DPF2 overexpression correlates with immune infiltration and dismal prognosis in hepatocellular carcinoma
- PMID: 39006087
- PMCID: PMC11242344
- DOI: 10.7150/jca.97437
DPF2 overexpression correlates with immune infiltration and dismal prognosis in hepatocellular carcinoma
Abstract
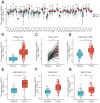
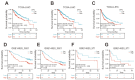
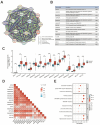
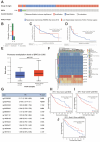
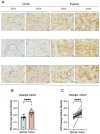
Background: Double plant homeodomain finger 2 (DPF2), belonging to the d4 family of structural domains, has been associated with various human malignancies. However, its impact on hepatocellular carcinoma (HCC) remains unclear. The objective of this study is to elucidate the role of DPF2 in the diagnosis and prognosis of HCC. Methods: DPF2 gene expression in HCC and adjacent tissues was analyzed using Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) databases, validated by immunohistochemical staining of Guangxi specimens and data from the Human Protein Atlas (HPA). Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genome (KEGG), and Gene Set Enrichment Analysis (GSEA) were used to identify DPF2's potential pathways and functions in HCC. DPF2's mutation and methylation statuses were assessed via cBioPortal and MethSurv. The association between DPF2 and immune infiltration was investigated by TIMER. The prognostic value of DPF2 in HCC was established through Kaplan-Meier and Cox regression analyses. Results: DPF2 levels were significantly higher in HCC than normal tissues (p<0.001), correlating with more severe HCC features (p<0.05). Higher DPF2 expression predicted poorer overall survival (OS), disease-specific survival (DSS), and progression-free interval (PFI). DPF2 involvement was noted in critical signaling pathways including the cell cycle and Wnt. It also correlated with T helper cells, Th2 cells, and immune checkpoints like CTLA-4, PD-1, and PD-L1. Conclusion: High DPF2 expression, associated with poor HCC prognosis, may disrupt tumor immune balance and promote immune evasion. DPF2 could potentially be utilized as a biomarker for diagnosing and prognosticating hepatocellular carcinoma.
Keywords: DPF2; hepatocellular carcinoma (HCC); immune infiltration; tumor prognosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Chhikara BS, Parang K. Global Cancer Statistics 2022: the trends projection analysis. Chemical Biology Letters. 2023;10:451. -
-
- Zhang C-H, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2029–41. - PubMed
-
- World Health Organization. GLOBOCAN 2020[EB/OL] 2020-10-01.
LinkOut - more resources
Full Text Sources
Research Materials

