A synbiotic mixture of Bifidobacterium breve M16-V, oligosaccharides and pectin, enhances Short Chain Fatty Acid production and improves lung health in a preclinical model for pulmonary neutrophilia
- PMID: 39006103
- PMCID: PMC11239554
- DOI: 10.3389/fnut.2024.1371064
A synbiotic mixture of Bifidobacterium breve M16-V, oligosaccharides and pectin, enhances Short Chain Fatty Acid production and improves lung health in a preclinical model for pulmonary neutrophilia
Abstract
Introduction: Pulmonary neutrophilia is a hallmark of numerous airway diseases including Chronic Obstructive Pulmonary Disease (COPD), Neutrophilic asthma, Acute Lung Injury (ALI), Acute Respiratory Distress Syndrome (ARDS) and COVID-19. The aim of the current study was to investigate the effect of dietary interventions on lung health in context of pulmonary neutrophilia.
Methods: Male BALB/cByJ mice received 7 intra-nasal doses of either a vehicle or lipopolysaccharides (LPS). To study the effect of nutritional interventions they received 16 intra-gastric doses of either a vehicle (PBS) or the following supplements (1) probiotic Bifidobacterium breve (B. breve) M16-V; (2) a prebiotic fiber mixture of short-chain galacto-oligosaccharides, long-chain fructo-oligosaccharides, and low-viscosity pectin in a 9:1:2 ratio (scGOS/lcFOS/lvPectin); and (3) A synbiotic combination B. breve M16-V and scGOS/lcFOS/lvPectin. Parameters for lung health included lung function, lung morphology and lung inflammation. Parameters for systemic immunomodulation included levels of fecal short chain fatty acids and regulatory T cells.
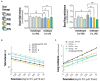
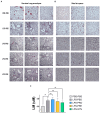
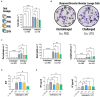
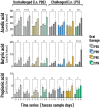
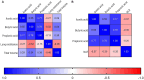
Results: The synbiotic supplement protected against the LPS induced decline in lung function (35% improved lung resistance at baseline p = 0.0002 and 25% at peak challenge, p = 0.0002), provided a significant relief from pulmonary neutrophilia (40.7% less neutrophils, p < 0.01) and improved the pulmonary neutrophil-to-lymphocyte ratio (NLR) by 55.3% (p = 0.0033). Supplements did not impact lung morphology in this specific experiment. LPS applied to the upper airways induced less fecal SCFAs production compared to mice that received PBS. The production of acetic acid between day -5 and day 16 was increased in all unchallenged mice (PBS-PBS p = 0.0003; PBS-Pro p < 0.0001; PBS-Pre, p = 0.0045; PBS-Syn, p = 0.0005) which upon LPS challenge was only observed in mice that received the synbiotic mixture of B. breve M16-V and GOS:FOS:lvPectin (p = 0.0003). A moderate correlation was found for butyric acid and lung function parameters and a weak correlation was found between acetic acid, butyric acid and propionic acid concentrations and NLR.
Conclusion: This study suggests bidirectional gut lung cross-talk in a mouse model for pulmonary neutrophilia. Neutrophilic lung inflammation coexisted with attenuated levels of fecal SCFA. The beneficial effects of the synbiotic mixture of B. breve M16-V and GOS:FOS:lvPectin on lung health associated with enhanced levels of SCFAs.
Keywords: Short Chain Fatty Acids; acetate; butyrate; gut-lung axis; neutrophil to lymphocyte ratio; nutraceuticals; pulmonary neutrophilia; synbiotics.
Copyright © 2024 Bezemer, Diks, Mortaz, van Ark, van Bergenhenegouwen, Kraneveld, Folkerts and Garssen.
Conflict of interest statement
JB and JG are both in part affiliated with Danone Nutricia Research BV, a company having commercial interest in dietary products. GB was in part employed by Impact Station. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

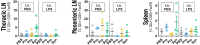
References
LinkOut - more resources
Full Text Sources
Miscellaneous

