Role of AURKB Inhibition in Reducing Proliferation and Enhancing Effects of Radiotherapy in Triple-Negative Breast Cancer
- PMID: 39006183
- PMCID: PMC11246031
- DOI: 10.2147/BCTT.S444965
Role of AURKB Inhibition in Reducing Proliferation and Enhancing Effects of Radiotherapy in Triple-Negative Breast Cancer
Abstract
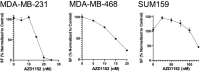
Breast cancer is a leading cause of cancer-related deaths in females. Triple-negative breast cancer (TNBC) subtype is the most aggressive form of breast cancer that lacks biomarkers and effective targeted therapies. Its high degree of heterogeneity as well as innate and acquired resistance to treatment creates further barriers in achieving positive clinical outcomes in TNBC. Thus, development of novel treatment approaches in TNBC is of high clinical significance. Multimodality approaches with targeted agents and radiotherapy (RT) are promising for increasing efficacy of treatment and circumventing resistance. Here we examined anticancer effects of the Aurora Kinase B (AURKB) inhibitor AZD1152 as a single agent and in combination with RT using various TNBC cell lines, MDA-MB-468, MDA-MB-231 and SUM-159. We observed that AZD1152 alone effectively inhibited colony formation in TNBC cell lines. The combination of AZD1152 at IC50 concentrations together with ionizing radiation further reduced colony formation as compared to the single agent treatment. Our data support the notion that inhibition of the AURKB pathway is a promising strategy for treatment and radiosensitization of TNBC and warrants further translational studies.
Keywords: AURKB; AZD1152; Aurora Kinase B; breast cancer; combination treatment; multimodality treatment; radiation.
Plain language summary
Breast cancer is a leading cause of cancer death in women globally. The triple negative breast cancer subtype confers the poorest oncologic outcomes and requires novel treatment approaches. Development of new therapeutics as well as combination treatments with radiation are crucial. Aurora Kinase B (AURKB) protein regulates cell division that is often altered in breast cancer, contributing to tumor pathogenesis. This study examined the combination of an AURKB inhibitor, AZD1152, with radiation therapy, compared to single-agent treatments, in treating triple negative breast cancer cells. Our results show that AZD1152 and ionizing radiation alone were able to delay cancer cell proliferation effectively. However, their combination further significantly inhibited cell proliferation compared to single-agent treatments. This suggests that further studies on this combination would be valuable in developing novel treatment strategies for breast cancer.
© 2024 Pellizzari et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures
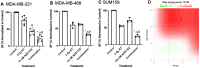
References
LinkOut - more resources
Full Text Sources
Miscellaneous

