Nalbuphine in Pediatric Emergence Agitation Following Cochlear Implantation: A Randomized Trial
- PMID: 39006192
- PMCID: PMC11244056
- DOI: 10.2147/DDDT.S451089
Nalbuphine in Pediatric Emergence Agitation Following Cochlear Implantation: A Randomized Trial
Abstract
Background: To investigate the effects of nalbuphine on emergency agitation (EA), which affects up to 80% of the children following otolaryngology procedures, in children undergoing cochlear implantation.
Methods: A prospective double-blinded randomized controlled clinical trial was conducted between November 2020 and October 2022. Eligible children, aged 6 months to 3 years old, were randomly assigned to either 0.1 mg/kg, 0.15 mg/kg, 0.2 mg/kg nalbuphine or 0.9% saline groups. EA was defined by the Pediatric Anesthesia Emergence Delirium (PAED) score ≥10. Extubation time, post-anesthesia care unit (PACU) length of stay, severe EA (PAED ≥ 15), peak PAED score, the Faces, Legs, Activity, Cry, and Consolability (FLACC) scale, Ramsay sedation score, and adverse events were also recorded.
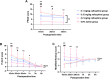
Results: A total of 104 children were enrolled, with 26 children in each group. Nalbuphine significantly reduced the EA occurrence from 73.1% in the saline group to 38.5%, 30.8%, and 26.9% in the 0.1 mg/kg, 0.15 mg/kg, and 0.2 mg/kg nalbuphine groups, respectively (P < 0.001), without affecting the extubation time and PACU length of stay. More children (34.6%) in the 0.9% saline group experienced severe EA. Higher dose nalbuphine (0.15 mg/kg, 0.2 mg/kg) showed lower peak PAED score, better analgesia and sedation effect compared with 0.1 mg/kg nalbuphine and saline groups. However, 0.2mg/kg nalbuphine caused undesired over-sedation in two (7.7%) children. No other adverse events were reported.
Conclusion: Young children undergoing cochlear implantation surgery were at a high risk of EA and postoperative pain, while 0.2 mg/kg nalbuphine might be an ideal candidate for EA and pain prevention when used under close monitoring.
Trial registration: ChiCTR2000040407.
Keywords: cochlear implantation; emergence agitation; nalbuphine; postoperative pain.
© 2024 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources