This is a preprint.
A Comprehensive Head-to-Head Comparison of Key Plasma Phosphorylated Tau 217 Biomarker Tests
- PMID: 39006421
- PMCID: PMC11245081
- DOI: 10.1101/2024.07.02.24309629
A Comprehensive Head-to-Head Comparison of Key Plasma Phosphorylated Tau 217 Biomarker Tests
Update in
-
A comprehensive head-to-head comparison of key plasma phosphorylated tau 217 biomarker tests.Brain. 2025 Feb 3;148(2):416-431. doi: 10.1093/brain/awae346. Brain. 2025. PMID: 39468767 Free PMC article.
Abstract
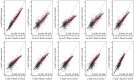
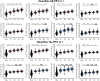
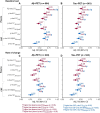
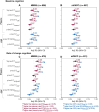
Plasma phosphorylated-tau 217 (p-tau217) is currently the most promising biomarkers for reliable detection of Alzheimer's disease (AD) pathology. Various p-tau217 assays have been developed, but their relative performance is unclear. We compared key plasma p-tau217 tests using cross-sectional and longitudinal measures of amyloid-β (Aβ)-PET, tau-PET, and cognition as outcomes, and benchmarked them against cerebrospinal fluid (CSF) biomarker tests. Samples from 998 individuals (mean[range] age 68.5[20.0-92.5], 53% female) from the Swedish BioFINDER-2 cohort were analyzed. Plasma p-tau217 was measured with mass spectrometry (MS) assays (the ratio between phosphorylated and non-phosphorylated [%p-tau217WashU]and ptau217WashU) as well as with immunoassays (p-tau217Lilly, p-tau217Janssen, p-tau217ALZpath). CSF biomarkers included p-tau217Lilly, and the FDA-approved p-tau181/Aβ42Elecsys and p-tau181Elecsys. All plasma p-tau217 tests exhibited high ability to detect abnormal Aβ-PET (AUC range: 0.91-0.96) and tau-PET (AUC range: 0.94-0.97). Plasma %p-tau217WashU had the highest performance, with significantly higher AUCs than all the immunoassays (P diff<0.007). For detecting Aβ-PET status, %p-tau217WashU had an accuracy of 0.93 (immunoassays: 0.83-0.88), sensitivity of 91% (immunoassays: 84-87%), and a specificity of 94% (immunoassays: 85-89%). Among immunoassays, p-tau217Lilly and plasma p-tau217ALZpath had higher AUCs than plasma p-tau217Janssen for Aβ-PET status (P diff<0.006), and p-tau217Lilly outperformed plasma p-tau217ALZpath for tau-PET status (P diff=0.025). Plasma %p-tau217WashU exhibited higher associations with all PET load outcomes compared to immunoassays; baseline Aβ-PET load (R2: 0.72; immunoassays: 0.47-0.58; Pdiff<0.001), baseline tau-PET load (R2: 0.51; immunoassays: 0.38-0.45; Pdiff<0.001), longitudinal Aβ-PET load (R2: 0.53; immunoassays: 0.31-0.38; Pdiff<0.001) and longitudinal tau-PET load (R2: 0.50; immunoassays: 0.35-0.43; Pdiff<0.014). Among immunoassays, plasma p-tau217Lilly was more strongly associated with Aβ-PET load than plasma p-tau217Janssen (P diff<0.020) and with tau-PET load than both plasma p-tau217Janssen and plasma p-tau217ALZpath (all P diff<0.010). Plasma %p-tau217 also correlated more strongly with baseline cognition (Mini-Mental State Examination[MMSE]) than all immunoassays (R2 %p-tau217WashU: 0.33; immunoassays: 0.27-0.30; P diff<0.024). The main results were replicated in an external cohort from Washington University in St Louis (n =219). Finally, p-tau217Nulisa showed similar performance to other immunoassays in subsets of both cohorts. In summary, both MS- and immunoassay-based p-tau217 tests generally perform well in identifying Aβ-PET, tau-PET, and cognitive abnormalities, but %p-tau217WashU performed significantly better than all the examined immunoassays. Plasma %p-tau217 may be considered as a stand-alone confirmatory test for AD pathology, while some immunoassays might be better suited as triage tests where positive results are confirmed with a second test.
Keywords: Alzheimer’s disease; CSF biomarkers; p-tau217; plasma biomarkers.
Conflict of interest statement
N.W, G.S, S.J, N.M.C, D.B, A.O.D, H.K, A.A, C.A.R, T.L.S.B, J.C.M, L.I, J.T, N.J.A., B.A, K.B, and A.P.B report no competing interests. N.R.B is co-inventor on a US patent application “Methods to detect novel tau species in CSF and use thereof to track tau neuropathology in Alzheimer’s disease and other tauopathies,” and “CSF phosphorylated tau and Amyloid beta profiles as biomarkers of tauopathies.” N.R.B is co-inventors on a non-provisional patent application “Methods of Diagnosing and Treating Based on Site-Specific Tau Phosphorylation.” NRB receives royalty income based on technology (blood plasma assay and methods of diagnosing AD with phosphorylation changes) licensed by Washington University to C2N Diagnostics. N.R.B. and R.J.B. are co-inventors on US patent applications: ‘Methods to detect novel tau species in CSF and use thereof to track tau neuropathology in Alzheimer’s disease and other tauopathies’ and ‘CSF phosphorylated tau and amyloid beta profiles as biomarkers of tauopathies’. G.T.B is an employee of Johnson and Johnson Innovative Medicine. S.E.S has received consultancy/speaker fees from Eisai, Eli Lilly, and Novo Nordisk. C.C has received research support from: GSK and EISAI. C.C is a member of the scientific advisory board of Circular Genomics and owns stocks. C.C is a member of the scientific advisory board of Admit. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche, and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). N.R.B. and R.J.B. are co-inventors on a non-provisional patent application: ‘Methods of diagnosing and treating based on site-specific tau phosphorylation’. R.J.B. co-founded C2N Diagnostics. Washington University and R.J.B. have equity ownership interest in C2N Diagnostics and receive royalty income based on technology (stable isotope labeling kinetics, blood plasma assay and methods of diagnosing Alzheimer’s disease with phosphorylation changes) that is licensed by Washington University to C2N Diagnostics. R.J.B. receives income from C2N Diagnostics for serving on the scientific advisory board. R.J.B. has received research funding from Avid Radiopharmaceuticals, Janssen, Roche/Genentech, Eli Lilly, Eisai, Biogen, AbbVie, Bristol Myers Squibb and Novartis. O.H. has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, C2N Diagnostics, Eli Lilly, Eisai, Fujirebio, GE Healthcare, and Roche. In the past 2 years, he has received consultancy/speaker fees from Alzpath, BioArctic, Biogen, Bristol Meyer Squibb, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens.
Figures

Similar articles
-
A comprehensive head-to-head comparison of key plasma phosphorylated tau 217 biomarker tests.Brain. 2025 Feb 3;148(2):416-431. doi: 10.1093/brain/awae346. Brain. 2025. PMID: 39468767 Free PMC article.
-
Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer's disease.Brain. 2023 Apr 19;146(4):1592-1601. doi: 10.1093/brain/awac333. Brain. 2023. PMID: 36087307 Free PMC article.
-
Comparison of plasma ALZpath p-Tau217 with Lilly p-Tau217 and p-Tau181 in a neuropathological cohort.Acta Neuropathol Commun. 2025 Jun 30;13(1):144. doi: 10.1186/s40478-025-02064-2. Acta Neuropathol Commun. 2025. PMID: 40588775 Free PMC article.
-
Plasma p-tau immunoassays in clinical research for Alzheimer's disease.Alzheimers Dement. 2025 Jan;21(1):e14397. doi: 10.1002/alz.14397. Epub 2024 Dec 3. Alzheimers Dement. 2025. PMID: 39625101 Free PMC article. Review.
-
Phosphorylated tau in Alzheimer's disease.Adv Clin Chem. 2023;116:31-111. doi: 10.1016/bs.acc.2023.05.001. Epub 2023 Jun 9. Adv Clin Chem. 2023. PMID: 37852722
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
