Rare HCV subtypes and retreatment outcomes in a cohort of European DAA-experienced patients
- PMID: 39006503
- PMCID: PMC11246049
- DOI: 10.1016/j.jhepr.2024.101072
Rare HCV subtypes and retreatment outcomes in a cohort of European DAA-experienced patients
Erratum in
-
Erratum regarding previously published articles.JHEP Rep. 2025 Feb 17;7(3):101359. doi: 10.1016/j.jhepr.2025.101359. eCollection 2025 Mar. JHEP Rep. 2025. PMID: 40170909 Free PMC article.
Abstract
Background and aims: Data on the prevalence and characteristics of so-called rare HCV genotypes (GTs) in larger cohorts is limited. This study investigates the frequency of rare GT and resistance-associated substitutions and the efficacy of retreatment in a European cohort.
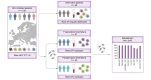
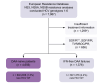
Methods: A total of 129 patients with rare GT1-6 were included from the European resistance database. NS3, NS5A, and NS5B were sequenced and clinical parameters and retreatment efficacies were collected retrospectively.
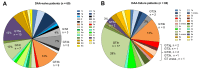
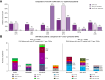
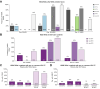
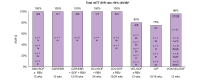
Results: Overall 1.5% (69/4,656) of direct-acting antiviral (DAA)-naive and 4.4% (60/1,376) of DAA-failure patients were infected with rare GT. Although rare GTs were almost equally distributed throughout GT1-6 in DAA-naive patients, we detected mainly rare GT4 (47%, 28/60 GT4; of these n = 17, subtype 4r) and GT3 (25%, 15/60 GT3, of these n = 8, subtype 3b) among DAA-failures. A total of 62% (37/60) of DAA failures had not responded to first-generation regimes and the majority was infected with rare GT4 (57%, 21/37). In contrast, among patients with failure to pangenotypic DAA regimens (38%, 23/60), infections with rare GT3 were overrepresented (57%, 13/23). Although NS5A RASs were uncommon in rare GT2, GT5a, and GT6, we observed combined RASs in rare GT1, GT3, and GT4 at positions 28, 30, 31, which can be considered as inherent. DAA failures with completed follow-up of retreatment, achieved a high SVR rate (94%, 45/48 modified intention-to-treat analysis; 92%, 45/49 intention-to-treat). Three patients with GT4f, 4r, or 3b, respectively, had virological treatment failure.
Conclusions: In this European cohort, rare HCV GT were uncommon. Accumulation of specific rare GT in DAA-failure patients suggests reduced antiviral activities of DAA regimens. The limited global availability of pangenotypic regimens for first line therapy as well as multiple targeted regimens for retreatment could result in HCV elimination targets being delayed.
Impact and implications: Data on the prevalence and characteristics of rare HCV genotypes (GT) in larger cohorts are still scarce. This study found low rates of rare HCV GTs among European HCV-infected patients. In direct-acting antiviral (DAA)-failure patients, rare GT3 subtypes accumulated after pangenotypic DAA treatment and rare GT4 after first generation DAA failure and viral resistance was detected at NS5A positions 28, 30, and 31. The limited global availability of pangenotypic DAA regimens for first line therapy as well as multiple targeted regimens for retreatment could result in HCV elimination targets being delayed.
Keywords: Direct-acting antivirals; Hepatitis C Virus; rare HCV genotypes; resistance-associated substitutions; treatment response.
© 2024 The Authors.
Conflict of interest statement
JD: research support from Gilead. CG: speaking and/or consulting fees from AbbVie and travel support from AbbVie and Gilead. CPB: speaking and/or consulting fees: AbbVie, BMS, Gilead, Merck/MSD. KP: no conflicts to disclose. KD: speaking and/or consulting fees: Gilead, AbbVie, Alnylam. PB: speaking and/or consulting fees: AbbVie, BMS, Falk, Gilead, Janssen, Merz Pharma, Merck/MSD. K-HP: no conflicts to disclose. JV: speaking and/or consulting fees from Abbott, AbbVie, Bristol-Myers, Squibb, Gilead, Medtronic, Merck/MSD and Roche. GD: speaking and/or consulting fees from Abbvie, Gilead. AG: advisor and steering committee member for AbbVie, Advanz, Albireo, Alexion, Astra Zeneca, Bayer, BMS, CSL Behring, Eisai, Gilead, Heel, Intercept, Ipsen, Merz, MSD, Novartis, Pfizer, Roche, Sanofi-Aventis and as speaker for AbbVie, Advanz, Alexion, BMS, Burgerstein, CSL Behring, Falk, Gilead, Intercept, Merz, MSD, Novartis, NovoNordisk, Roche; research support from Intercept and Falk (NAFLD CSG), Novartis. FPR: honoraria for lectures, consulting activities and travel support from the Falk Foundation, AbbVie, Gilead, Ipsen, Astra Zeneca, Roche and Novartis. TB: speaking and/or consulting fees or travel support from Abbvie, Gilead, SOBI, CSL Behring, Merck, Gore, Advanz. JMS: consultant: Akero, Alentis Therapeutics, Astra Zeneca, Apollo Endosurgery, 89Bio, Boehringer Ingelheim, GSK, Ipsen, Inventiva Pharma, Madrigal, MSD, Northsea Therapeutics, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, Siemens Healthineers. Research Funding: Boehringer Ingelheim, Siemens Healthcare GmbH. Stock Options: AGED diagnostics, Hepta Bio. Speaker Honorarium: Gilead Sciences, Advanz, Echosens, MedPublico GmbH. ED: speaking fees: Abbvie, Gilead. TG: advisory board for GoLiver Therapeutics. CM: speaking and/or consulting fees from Abbvie, Gilead, MSD, Intercept. Research support: Abbvie, Gilead, MSD, Intercept. JT: speaking and/or consulting fees from Abbvie, Gilead, Viiv. TD: Speaking and/or consulting fees: Abbvie, BMS, Gilead, MSD, Roche. JF: no conflicts to disclose. TB: Speaking and/or consulting fees: AbbVie, Alexion, Bayer, Boehringer Ingelheim, BMS, Gilead, GSK, Intercept, Janssen, MSD/Merck, Merz, Novartis, Sequana Medical and Roche. Research support: AbbVie, Roche, BMS, Gilead, Novartis, Merck/MSD, Intercept, Janssen, Novartis, Sequana Medical, and Pfizer. AEK: speaking and/or consulting fees: Abbvie, Advanz, Alentis, AlphaSigma, AOP Orphan, AstraZenca, Avior, Bayer, BMS, CMS, CymaBay, Eisai, Escient, Falk, Gilead, GSK, Guidepoint, Intercept, Ipsen, Lilly, Medscape, Mirum, MSD, Myr, Novartis, Roche, Takeda, Viofor, Zambon. Research support: Intercept, Gilead. BM: speaking and/or consulting fees: Merck/MSD, AbbVie, Intercept, Astra, Bayer, BMS, Gilead. Research support: Gilead. SZ: consultancy and/or speaker's bureau: Abbvie, BioMarin, Boehringer Ingelheim, Gilead, GSK, Ipsen, Madrigal, MSD/Merck, NovoNordisk, and SoBi. CS: speaking and/or consulting fees: AbbVie, Gilead, Merck/MSD. Research support: AbbVie, Gilead.
Figures
References
-
- WHO . 2024. Hepatitis C fact sheet.https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
-
- Pawlotsky J.M. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology. 2016;151:70–86. - PubMed
-
- Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol. 2016;64:486–504. - PubMed
-
- Sarrazin C. Treatment failure with DAA therapy: importance of resistance. J Hepatol. 2021;74:1472–1482. - PubMed
-
- Fahnøe U., Pedersen M.S., Sølund C., et al. Global evolutionary analysis of chronic hepatitis C patients revealed significant effect of baseline viral resistance, including novel non-target sites, for DAA-based treatment and retreatment outcome. J Viral Hepat. 2021;28:302–316. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

