Emerging applications of hypomethylating agents in the treatment of glioblastoma (Review)
- PMID: 39006906
- PMCID: PMC11240870
- DOI: 10.3892/mco.2024.2757
Emerging applications of hypomethylating agents in the treatment of glioblastoma (Review)
Abstract
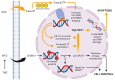
DNA hypomethylating agents (HMAs) such as decitabine and 5-azacytidine have established roles in the treatment paradigms for myelodysplastic syndrome and acute myelogenous leukemia, where they are considered to exert their anticancer effects by restoring the expression of tumor suppressor genes. Due to their relatively favorable adverse effect profile and known ability to pass through the blood-brain barrier, applications in the treatment of glioblastoma (GBM) and other central nervous system malignancies are under active investigation. The present review examines the types of HMAs currently available, their known and less-understood antineoplastic mechanisms, and the evidence to date of their preclinical and clinical efficacy in glioblastoma and other solid malignancies. The present review discusses the potential synergies HMAs may have with established and emerging GBM treatments, including temozolomide, immune checkpoint inhibitors and cancer vaccines. Recent successes and setbacks in clinical trials for newly diagnosed and recurrent GBM are summarized in order to highlight opportunities for HMAs to improve therapeutic responses. Challenges for future clinical trials are also assessed.
Keywords: decitabine; glioblastoma; hypomethylating agent; immunotherapy; temozolomide.
Copyright: © 2024 Silva-Hurtado et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Hypomethylating Agents and Immunotherapy: Therapeutic Synergism in Acute Myeloid Leukemia and Myelodysplastic Syndromes.Front Oncol. 2021 Feb 25;11:624742. doi: 10.3389/fonc.2021.624742. eCollection 2021. Front Oncol. 2021. PMID: 33718188 Free PMC article. Review.
-
The double-edged sword of (re)expression of genes by hypomethylating agents: from viral mimicry to exploitation as priming agents for targeted immune checkpoint modulation.Cell Commun Signal. 2017 Mar 31;15(1):13. doi: 10.1186/s12964-017-0168-z. Cell Commun Signal. 2017. PMID: 28359286 Free PMC article. Review.
-
Different mechanisms of drug resistance to hypomethylating agents in the treatment of myelodysplastic syndromes and acute myeloid leukemia.Drug Resist Updat. 2022 Mar;61:100805. doi: 10.1016/j.drup.2022.100805. Epub 2022 Jan 21. Drug Resist Updat. 2022. PMID: 35227933 Review.
-
Mechanisms of Action of Hypomethylating Agents: Endogenous Retroelements at the Epicenter.Front Oncol. 2021 Mar 9;11:650473. doi: 10.3389/fonc.2021.650473. eCollection 2021. Front Oncol. 2021. PMID: 33768008 Free PMC article. Review.
-
Clinical activity, pharmacokinetics, and pharmacodynamics of oral hypomethylating agents for myelodysplastic syndromes/neoplasms and acute myeloid leukemia: A multidisciplinary review.J Oncol Pharm Pract. 2024 Jun;30(4):721-736. doi: 10.1177/10781552241238979. Epub 2024 Mar 21. J Oncol Pharm Pract. 2024. PMID: 38509812 Free PMC article. Review.
Cited by
-
Cellular heterogeneity and therapeutic response profiling of human IDH+ glioma stem cell cultures.bioRxiv [Preprint]. 2025 Aug 1:2025.07.29.667532. doi: 10.1101/2025.07.29.667532. bioRxiv. 2025. PMID: 40766425 Free PMC article. Preprint.
References
Publication types
LinkOut - more resources
Full Text Sources
