Architecture design and advanced manufacturing of heart-on-a-chip: scaffolds, stimulation and sensors
- PMID: 39006908
- PMCID: PMC11239895
- DOI: 10.1038/s41378-024-00692-7
Architecture design and advanced manufacturing of heart-on-a-chip: scaffolds, stimulation and sensors
Abstract
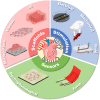
Heart-on-a-chip (HoC) has emerged as a highly efficient, cost-effective device for the development of engineered cardiac tissue, facilitating high-throughput testing in drug development and clinical treatment. HoC is primarily used to create a biomimetic microphysiological environment conducive to fostering the maturation of cardiac tissue and to gather information regarding the real-time condition of cardiac tissue. The development of architectural design and advanced manufacturing for these "3S" components, scaffolds, stimulation, and sensors is essential for improving the maturity of cardiac tissue cultivated on-chip, as well as the precision and accuracy of tissue states. In this review, the typical structures and manufacturing technologies of the "3S" components are summarized. The design and manufacturing suggestions for each component are proposed. Furthermore, key challenges and future perspectives of HoC platforms with integrated "3S" components are discussed. Architecture design concepts of scaffolds, stimulation and sensors in chips.
Keywords: Biosensors; Electrical and electronic engineering; Microfluidics.
© The Author(s) 2024.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures







References
Publication types
LinkOut - more resources
Full Text Sources
