Isolation of Caribbean Ciguatoxin-5 (C-CTX5) and confirmation of its structure by NMR spectroscopy
- PMID: 39006909
- PMCID: PMC11238948
- DOI: 10.1016/j.tet.2024.134115
Isolation of Caribbean Ciguatoxin-5 (C-CTX5) and confirmation of its structure by NMR spectroscopy
Abstract
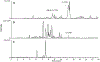
Ciguatera poisoning occurs throughout subtropical and tropical regions globally. The Virgin Islands in the Caribbean Sea is a known hyperendemic region for ciguatera and has been associated with Caribbean ciguatoxin (C-CTX) contamination in fish. An algal C-CTX (C-CTX5) was identified in Gambierdiscus silvae and G. caribeaus isolated from benthic algal samples collected in waters south St. Thomas, US Virgin Islands. The highest CTX-producing isolate, G. silvae 1602 SH-6, was grown at large-scale to isolate sufficient C-CTX5 for structural confirmation by NMR spectroscopy. A series of orthogonal extraction and fractionation procedures resulted in purification of approximately 40 μg of C-CTX5, as estimated by quantitative NMR. A suite of 1D and 2D NMR experiments were acquired that verified the structure originally proposed for C-CTX5. The structural confirmation and successful isolation of C-CTX5 opens the way for work on the stability, toxicology and biotransformation of C-CTXs, as well as for the production of quantitative reference materials for analytical method development and validation. The strategies developed for purification of C-CTX5 may also apply to isolation and purification of CTXs from the Pacific Ocean and other regions.
Keywords: Caribbean ciguatoxin; Ciguatera; LC−HRMS; NMR; structure confirmation.
Conflict of interest statement
Conflict of Interest Statement The authors declare no conflict of interest.
Figures

References
-
- Beach DG, Kerrin ES, McCarron P, Kilcoyne J, Giddings SD, Waaler T, Rundberget T, Samdal IA, Løvberg KE, Miles CO, 2020. Boronate techniques for clean-up and concentration of the vic-diol-containing tetrodotoxins and azaspiracids from shellfish, in: Hess P. (Ed.), Harmful Algae 2018 – From Ecosystems to Socioecosystems. Proceedings of the 18th Intl. Conf. on Harmful Algae. International Society for the Study of Harmful Algae, Nantes France, pp. 125–128.
-
- Burton IW, Quilliam MA, Walter JA, 2005. Quantitative 1H NMR with external standards: use in preparation of calibration solutions for algal toxins and other natural products. Anal. Chem. 77, 3123–3131. - PubMed
-
- Estevez P, Castro D, Manuel Leao J, Yasumoto T, Dickey R, Gago-Martinez A, 2019. Implementation of liquid chromatography tandem mass spectrometry for the analysis of ciguatera fish poisoning in contaminated fish samples from Atlantic coasts. Food Chem. 280, 8–14. - PubMed
-
- Estevez P, Leao JM, Yasumoto T, Dickey RW, Gago-Martinez A, 2020a. Caribbean ciguatoxin-1 stability under strongly acidic conditions: characterisation of a new C-CTX1 methoxy congener. Food Addit. Contam. 37, 519–529. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

