Transcriptomic investigations of polymyxins and colistin/sulbactam combination against carbapenem-resistant Acinetobacter baumannii
- PMID: 39006922
- PMCID: PMC11245955
- DOI: 10.1016/j.csbj.2024.05.043
Transcriptomic investigations of polymyxins and colistin/sulbactam combination against carbapenem-resistant Acinetobacter baumannii
Abstract
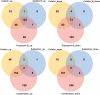
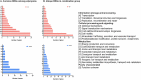
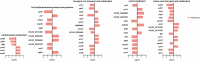
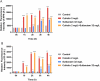
Carbapenem-resistant Acinetobacter baumannii (CRAB) is a Priority 1 (Critical) pathogen urgently requiring new antibiotics. Polymyxins are a last-line option against CRAB-associated infections. This transcriptomic study utilized a CRAB strain to investigate mechanisms of bacterial killing with polymyxin B, colistin, colistin B, and colistin/sulbactam combination therapy. After 4 h of 2 mg/L polymyxin monotherapy, all polymyxins exhibited common transcriptomic responses which primarily involved disruption to amino acid and fatty acid metabolism. Of the three monotherapies, polymyxin B induced the greatest number of differentially expressed genes (DEGs), including for genes involved with fatty acid metabolism. Gene disturbances with colistin and colistin B were highly similar (89 % common genes for colistin B), though effects on gene expression were generally lower (0-1.5-fold in most cases) with colistin B. Colistin alone (2 mg/L) or combined with sulbactam (64 mg/L) resulted in rapid membrane disruption as early as 1 h. Transcriptomic analysis of this combination revealed that the effects were driven by colistin, which included disturbances in fatty acid synthesis and catabolism, and inhibition of nutrient uptake. Combination therapy produced substantially higher fold changes in 72 % of DEGs shared with monotherapy, leading to substantially greater reductions in fatty acid biosynthesis and increases in biofilm, cell wall, and phospholipid synthesis. This indicates synergistic bacterial killing with the colistin/sulbactam combination results from a systematic increase in perturbation of many genes associated with bacterial metabolism. These mechanistic insights enhance our understanding of bacterial responses to polymyxin mono- and combination therapy and will assist to optimize polymyxin use in patients.
Keywords: Carbapenem-resistant Acinetobacter baumannii; Combination; Polymyxins; Sulbactam; Transcriptomics.
© 2024 The Authors.
Conflict of interest statement
None.
Figures





References
-
- World Health Organization WHO publishes list of bacteria for which new antibiotics are urgently needed. Neurosciences. 2017;22:159–160.
-
- CHINET. 2021. 〈http://www.chinets.com/〉.
-
- Opal S.M., Calandra T. Antibiotic usage and resistance: gaining or losing ground on infections in critically ill patients? JAMA. 2009;302:2367–2368. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
