Temozolomide based treatment in glioblastoma: 6 vs. 12 months
- PMID: 39006948
- PMCID: PMC11240269
- DOI: 10.3892/ol.2024.14551
Temozolomide based treatment in glioblastoma: 6 vs. 12 months
Abstract
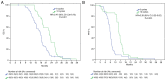
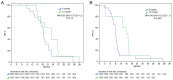
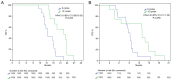
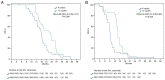
The Stupp regimen remains the standard treatment for newly diagnosed glioblastomas, although the prognosis remains poor. Several temozolomide alternative schedules have been studied, with extended adjuvant treatment (>6 cycles of temozolomide) frequently used, although different trials have indicated contrasting results. Survival data of 87 patients who received 6 ('6C' group) or 12 ('12C' group) cycles of temozolomide were collected between 2012 and 2022. A total of 45 patients were included in the 6C group and 42 patients were included in the 12C group. Data on isocitrate dehydrogenase mutation and methylguanine-DNA-methyltransferase (MGMT) promoter methylation status were also collected. The 12C group exhibited statistically significantly improved overall survival [OS; 22.8 vs. 17.5 months; hazard ratio (HR), 0.47; 95% CI, 0.30-0.73; P=0.001] and progression-free survival (15.3 vs. 9 months; HR, 0.39; 95% CI, 0.25-0.62; P=0.001). However, in the subgroup analysis according to MGMT status, OS in the 12C group was significantly superior to OS in the 6C group only in the MGMT unmethylated tumors. The present data suggested that extended adjuvant temozolomide appeared to be more effective than the conventional six cycles.
Keywords: Stupp; adjuvant therapy; extended temozolomide; glioblastoma.
Copyright: © 2024 Fasano et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
