Artemisia smithii patches form fertile islands and lead to heterogeneity of soil bacteria and fungi within and around the patches in alpine meadows of the Qinghai-Tibetan Plateau
- PMID: 39006955
- PMCID: PMC11239433
- DOI: 10.3389/fpls.2024.1411839
Artemisia smithii patches form fertile islands and lead to heterogeneity of soil bacteria and fungi within and around the patches in alpine meadows of the Qinghai-Tibetan Plateau
Abstract
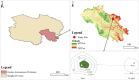
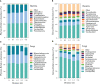
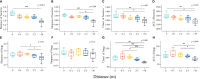
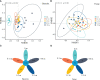
Herbivore-avoided plant patches are one of the initial characteristics of natural grassland degradation. These vegetation patches can intensify the spatial heterogeneity of soil nutrients within these grasslands. However, the effects of non-edible plant patches patches on the spatial heterogeneity of microorganisms have not been sufficiently studied in alpine meadows of the Qinghai-Tibetan Plateau, especially patches formed by herbaceous plants. To answer this question, soil nutrients, plant assembly, and microbial communities were measured inside, around, and outside of Artemisia smithii patches. These were 0 m (within the patch), 0-1 m (one meter from the edge of the patch), 1-2 m (two meters from the edge of the patch), 2-3 m (three meters from the edge of the patch), and >30 m (non-patch grassland more than thirty meters from the edge of the patch). Our results showed that A. smithii patches accumulated more aboveground biomass (AGB) within the patches (0 m), and formed fertile islands with the soil around the patches. Additionally, A. smithii patches increased soil bacterial diversity within (0 m) and around (0-1 m) the patches by primarily enriching copiotrophic bacteria (Actinobacteria), while the diversity of fungal communities increased mainly in the 0-1 m area but not within the patches. Bacterial community diversity was driven by pH, urease, nitrate nitrogen (NO3 --N), and microbial biomass carbon (MBC). The contents of soil water (SWC), soil organic matter (SOM), urease, NO3 --N, and MBC were the main factors influencing the diversity of the fungal community. This study elucidates the vegetation, nutrients, and microbial heterogeneity and their interrelationships, which are observed in fertile islands of herbivore-avoided plant patches in alpine meadows, and provides further insights into the spatial pattern of nutrients in patchy degraded grasslands.
Keywords: Artemisia smithii; alpine meadows; fertile islands; herbivore-avoided plant patches; heterogeneous distribution; soil microorganism.
Copyright © 2024 Yang, Yu, Song and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Abalori T. A., Cao W., Atogi-Akwoa Weobong C., Sam F. E., Li W., Osei R., et al. (2022). Effects of vegetation patchiness on ecosystem carbon and nitrogen storage in the alpine grassland of the Qilian Mountains. Front. Env. Sci. 10. doi: 10.3389/fenvs.2022.879717 - DOI
-
- Berg N., Unc A., Steinberger Y. (2015). Examination of biotic and abiotic controls of soil bacterial diversity under perennial shrubs in xeric soils. Catena 127, 124–128. doi: 10.1016/j.catena.2014.12.029 - DOI
-
- Bremner J. M., Mulvaney C. (1982). “Nitrogen-total. Methods of soil analysis,” in Part 2. Chemical and microbiological properties. Eds. Page A. L., Miller D. R., Keeney D. R. (America Soceity of Agronomy, Soil Science Society of America, Madison WI: ), 595–624.
-
- Brookes P., Landman A., Pruden G., Jenkinson D. (1985). Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 17, 837–842. doi: 10.1016/0038-0717(85)90144-0 - DOI
LinkOut - more resources
Full Text Sources

