Comparative effect of skeletal stem cells versus bone marrow mesenchymal stem cells on rotator cuff tendon-bone healing
- PMID: 39007033
- PMCID: PMC11245954
- DOI: 10.1016/j.jot.2024.05.005
Comparative effect of skeletal stem cells versus bone marrow mesenchymal stem cells on rotator cuff tendon-bone healing
Abstract
Background: Bone marrow mesenchymal stem cells (BMSCs) have immense potential in applications for the enhancement of tendon-bone (T-B) healing. Recently, it has been well-reported that skeletal stem cells (SSCs) could induce bone and cartilage regeneration. Therefore, SSCs represent a promising choice for cell-based therapies to improve T-B healing. In this study, we aimed to compare the therapeutic potential of SSCs and BMSCs for tendon-bone healing.
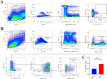
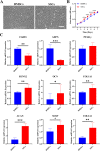
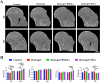
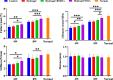
Methods: SSCs and BMSCs were isolated by flow cytometry, and their proliferation ability was measured by CCK-8 assay. The osteogenic, chondrogenic, and adipogenic gene expression in cells was detected by quantitative real-time polymerase chain reaction (qRT-PCR). C57BL/6 mice underwent unilateral supraspinatus tendon detachment and repair, and the mice were then randomly allocated to 4 groups: control group (tendon-bone interface without any treatment), hydrogel group (administration of blank hydrogel into the tendon-bone interface), hydrogel + BMSCs group (administration of hydrogel with BMSCs into the tendon-bone interface), and hydrogel + SSCs group (administration of hydrogel with SSCs into the tendon-bone interface). Histological staining, Micro-computed tomography (Micro-CT) scanning, biomechanical testing, and qRT-PCR were performed to assay T-B healing at 4 and 8 weeks after surgery.
Results: SSCs showed more cell proportion, exhibited stronger multiplication capacity, and expressed higher osteogenic and chondrogenic markers and lower adipogenic markers than BMSCs. In vivo assay, the SSCs group showed a better-maturated interface which was characterized by richer chondrocytes and more proteoglycan deposition, as well as more newly formed bone at the healing site and increased mechanical properties when compared to other there groups. qRT-PCR analysis revealed that the healing interface in the SSCs group expressed more transcription factors essential for osteogenesis and chondrogenesis than the interfaces in the other groups.
Conclusions: Overall, the results demonstrated the superior therapeutic potential of SSCs over BMSCs in tendon-bone healing.
The translational potential of this article: This current study provides valuable insights that SSCs may be a more effective cell therapy for enhancing T-B healing compared to BMSCs.
Keywords: Bone marrow mesenchymal stem cells; Cell-based therapies; Skeletal stem cells; Tendon-bone healing; Therapeutic potential.
© 2024 The Authors.
Figures




References
-
- Zhang T., Li S., Chen Y., Xiao H., Wang L., Hu J., et al. Characterize the microstructure change after tendon enthesis injury using synchrotron radiation μCT. J Orthop Res. 2022;40(11):2678–2687. [eng] - PubMed
-
- Yea J.-H., Bae T.S., Kim B.J., Cho Y.W., Jo C.H. Regeneration of the rotator cuff tendon-to-bone interface using umbilical cord-derived mesenchymal stem cells and gradient extracellular matrix scaffolds from adipose tissue in a rat model. Acta Biomater. 2020;114:104–116. [eng] - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

