Upregulation of FABP4 induced inflammation in the pathogenesis of chronic tendinopathy
- PMID: 39007036
- PMCID: PMC11245957
- DOI: 10.1016/j.jot.2024.06.007
Upregulation of FABP4 induced inflammation in the pathogenesis of chronic tendinopathy
Abstract
Objectives: Excessive inflammation contributes to the pathogenesis of tendinopathy. Fatty acid binding protein 4 (FABP4) is a pro-inflammatory adipokine mediating various metabolic and inflammatory diseases. This study aimed to examine the expression of FABP4 and its association with the expressions of inflammatory cytokines in tendinopathy. The effects of a single injection of FABP4 on tendon pathology and inflammation were examined. The effect of FABP4 on the expressions of inflammatory cytokines and the effect of IL-1β on the expression of FABP4 in tendon-derived stem/progenitor cells (TDSCs) were also investigated.
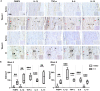
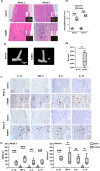
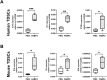
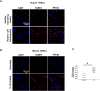
Methods: 1) Clinical patellar tendinopathy samples, healthy hamstring tendon samples, and healthy patellar tendon samples, 2) rotator cuff tendinopathy samples and healthy hamstring tendon samples; and 3) Achilles tendons of mice after saline or collagenase injection (CI) were stained for FABP4, IL-1β, IL-6, TNF-α and IL-10 by immunohistochemistry (IHC). For the rotator cuff tendinopathy samples, co-localization of FABP4 with IL-1β and TNF-α was done by immunofluorescent staining (IF). Mouse Achilles tendons injected with FABP4 or saline were collected for histology and IHC as well as microCT imaging post-injection. TDSCs were isolated from human and mouse tendons. The mRNA expressions of inflammatory cytokines in human and mouse TDSCs after the addition of FABP4 was quantified by qRT-PCR. The expression of FABP4 in TDSCs isolated from rotator cuff tendinopathy samples and healthy hamstring tendon samples was examined by IF. Mouse Achilles TDSCs were treated with IL-1β. The mRNA and protein expressions of FABP4 were examined by qRT-PCR and IF, respectively.
Results: There was significant upregulation of FABP4 in the patellar tendinopathy samples and rotator cuff tendinopathy samples compared to their corresponding controls. FABP4 was mainly expressed in the pathological areas including blood vessels, hypercellular and calcified regions. The expressions of IL-1β and TNF-α increased in human rotator cuff tendinopathy samples and co-localized with the expression of FABP4. Collagenase induced tendinopathic-like histopathological changes and ectopic calcification in the mouse Achilles tendinopathy model. The expressions of inflammatory cytokines (IL-1β, IL-6, TNF-α, IL-10) and FABP4 increased in hypercellular region, round cells chondrocyte-like cells and calcified regions in the mouse Achilles tendons post-collagenase injection. A single injection of FABP4 in mouse Achilles tendons induced histopathological changes resembling tendinopathy, with increased cell rounding, loss of collagen fiber alignment, and additionally presence of chondrocyte-like cells and calcification post-injection. The expressions of IL1-β, IL-6, TNF-α and IL-10 increased in mouse Achilles tendons post-FABP4 injection. FABP4 increased the expressions of IL10, IL6, and TNFa in human TDSCs as well as the expressions of Il1b, Il6, and Il10 in mouse TDSCs. Human tendinopathy TDSCs expressed higher level of FABP4 compared to healthy hamstring TDSCs. Besides, IL-1β increased the expression of FABP4 in mouse TDSCs.
Conclusion: In conclusion, an upregulation of FABP4 is involved in excessive inflammation and pathogenesis of tendinopathy. TDSCs is a potential source of FABP4 during tendon inflammation.
Translation potential of this article: FABP4 can be a potential treatment target of tendinopathy.
Keywords: Collagenase-induced tendon injury; FABP4; Inflammation; Tendinopathy; Tendon-derived stem / progenitor cells.
© 2024 The Authors.
Conflict of interest statement
We don't any potential conflicts of interest.
Figures





References
-
- Hu J.J., Yin Z., Shen W.L., Xie Y.B., Zhu T., Lu P., et al. Pharmacological regulation of in situ tissue stem cells differentiation for soft tissue calcification treatment. Stem Cell. 2016;34(4):1083–1096. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

