Outcomes of patients with advanced solid tumors who discontinued immune-checkpoint inhibitors: a systematic review and meta-analysis
- PMID: 39007061
- PMCID: PMC11245998
- DOI: 10.1016/j.eclinm.2024.102681
Outcomes of patients with advanced solid tumors who discontinued immune-checkpoint inhibitors: a systematic review and meta-analysis
Abstract
Background: The outcome of patients with metastatic tumors who discontinued immune checkpoint inhibitors (ICIs) not for progressive disease (PD) has been poorly explored. We performed a meta-analysis of all studies reporting the clinical outcome of patients who discontinued ICIs for reasons other than PD.
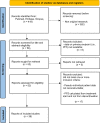
Methods: We searched PubMed, Embase and Scopus databases, from the inception of each database to December 2023, for clinical trials (randomized or not) and observational studies assessing PD-(L)1 and CTLA-4 inhibitors in patients with metastatic solid tumors who discontinued treatment for reasons other than PD. Each study had to provide swimmer plots or Kaplan-Meier survival curves enabling the reconstruction of individual patient-level data on progression-free survival (PFS) following the discontinuation of immunotherapy. The primary endpoint was PFS from the date of treatment discontinuation overall and according to tumor histotype, type of treatment and reason of discontinuation. The Combersure's method was used to estimate meta-analytical non-parametric summary survival curves assuming random effects at study level.
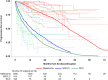
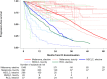
Findings: Thirty-six studies (2180 patients) were included. The pooled median PFS (mPFS) was 24.7 months (95% CI, 18.8-30.6) and the PFS-rate at 12, 24, and 36 months was respectively 69.8% (95% CI, 63.1-77.3), 51.0% (95% CI, 43.4-59.8) and 34.0% (95% CI, 27.0-42.9). Univariable analysis showed that the mPFS was significantly longer for patients with melanoma (43.0 months), as compared with non-small cell lung cancer (NSCLC, 13.5 months) and renal cell carcinoma (RCC, 10.0 months; between-strata comparison test p-value < 0.001); for patients treated with anti-PD-(L)1 + anti-CTLA-4 as compared with anti-PD-(L)1 monotherapy (44.6 versus 19.9 months; p-value < 0.001), and in NSCLC when the reason of treatment discontinuation was elective as compared with toxicity onset (19.6 versus 4.8 months; p-value = 0.003). The multivariable analysis confirmed these differences.
Interpretation: The long-term outcome of patients who stopped ICIs for reasons other than PD was substantially affected by clinicopathological features: PFS after treatment discontinuation was longer in patients with melanoma, and/or treated with anti-PD-(L)1 + anti-CTLA-4, and shorter in patients with RCC or in those patients with NSCLC who stopped treatment for toxicity onset.
Funding: The Italian Ministry of University and Research (PRIN 2022Y7HHNW).
Keywords: Discontinuation; Immune-checkpoint inhibitors; Meta-analysis melanoma; NSCLC.
© 2024 The Authors.
Conflict of interest statement
LP declares speaker engagements with Pierre Fabre. TDP declares Advisory board/Consultations roles with GSK Boehringer Ingelheim and trial support from Pfizer Blueprint Medicines Gilead Amgen Merck. CC declares travel supports from Astra Zeneca and Roche. GLC declares advisory roles and speaker engagements with Novocure, Bristol-Myers Squibb, Astrazeneca, Novartis, Merck Sharp & Dohme, Bayer, and Astellas. GV declares receipt of grants/research supports from Roche/Genentech, Ventana Medical Systems, Dako/Agilent Technologies: receipt of honoraria or consultation fees from Ventana, Dako/Agilent, Roche, MSD Oncology, AstraZeneca, Daiichi Sankyo, Pfizer, Gilead. RDG reports that his institutions receive support for his salary from AstraZeneca, Roche and Merck. AM declares to receive royalties for reagents related to innate immunity; consultant/advisory board roles for Novartis, Roche, Ventana, Pierre Fabre, Verily, Abbvie, BMS, J&J, Imcheck, Myeloid Therapeutics, Astra Zeneca, Biovelocita, BG Fund, Third Rock Venture, Biolegend Verseau Therapeutics, Macrophage pharma, Ellipses Pharma, Olatec Therapeutics, Moderna, Henlius.
Figures


References
-
- Hirsch I., Goldstein D.A., Tannock I.F., et al. Optimizing the dose and schedule of immune checkpoint inhibitors in cancer to allow global access. Nat Med. 2022;28:2236–2237. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

