CD39 delineates chimeric antigen receptor regulatory T cell subsets with distinct cytotoxic & regulatory functions against human islets
- PMID: 39007132
- PMCID: PMC11239501
- DOI: 10.3389/fimmu.2024.1415102
CD39 delineates chimeric antigen receptor regulatory T cell subsets with distinct cytotoxic & regulatory functions against human islets
Abstract
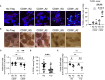
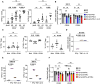
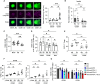
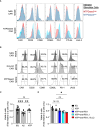
Human regulatory T cells (Treg) suppress other immune cells. Their dysfunction contributes to the pathophysiology of autoimmune diseases, including type 1 diabetes (T1D). Infusion of Tregs is being clinically evaluated as a novel way to prevent or treat T1D. Genetic modification of Tregs, most notably through the introduction of a chimeric antigen receptor (CAR) targeting Tregs to pancreatic islets, may improve their efficacy. We evaluated CAR targeting of human Tregs to monocytes, a human β cell line and human islet β cells in vitro. Targeting of HLA-A2-CAR (A2-CAR) bulk Tregs to HLA-A2+ cells resulted in dichotomous cytotoxic killing of human monocytes and islet β cells. In exploring subsets and mechanisms that may explain this pattern, we found that CD39 expression segregated CAR Treg cytotoxicity. CAR Tregs from individuals with more CD39low/- Tregs and from individuals with genetic polymorphism associated with lower CD39 expression (rs10748643) had more cytotoxicity. Isolated CD39- CAR Tregs had elevated granzyme B expression and cytotoxicity compared to the CD39+ CAR Treg subset. Genetic overexpression of CD39 in CD39low CAR Tregs reduced their cytotoxicity. Importantly, β cells upregulated protein surface expression of PD-L1 and PD-L2 in response to A2-CAR Tregs. Blockade of PD-L1/PD-L2 increased β cell death in A2-CAR Treg co-cultures suggesting that the PD-1/PD-L1 pathway is important in protecting islet β cells in the setting of CAR immunotherapy. In summary, introduction of CAR can enhance biological differences in subsets of Tregs. CD39+ Tregs represent a safer choice for CAR Treg therapies targeting tissues for tolerance induction.
Keywords: Treg-regulatory T cell; chimeric antigen receptor; cytotoxicity; immunoregulation; type 1 diabetes.
Copyright © 2024 Wu, Chen, Whitener, MacDougall, Coykendall, Yan, Kim, Harper, Pathak, Iliopoulou, Hestor, Saunders, Spears, Sévigny, Maahs, Basina, Sharp, Gloyn, Powers, Kim, Jensen and Meyer.
Conflict of interest statement
EM: Sponsored research funding and scientific advisory Orca Biosciences, Scientific Advisory and equity holder, Tract Therapeutics, Indee Labs, Jura Biosciences, Saffron Therapeutics. Scientific Advisory CTI, Kyverna. KJ is an investor equity holder and Scientific Advisor Consultant to Deka Biosciences. MM is scientific advisory board member, consultant, and equity holder in Syntax Bio. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

