Targeting apoptotic pathways for cancer therapy
- PMID: 39007268
- PMCID: PMC11245162
- DOI: 10.1172/JCI179570
Targeting apoptotic pathways for cancer therapy
Erratum in
-
Corrigendum to Targeting apoptotic pathways for cancer therapy.J Clin Invest. 2025 Jul 15;135(14):e196275. doi: 10.1172/JCI196275. eCollection 2025 Jul 15. J Clin Invest. 2025. PMID: 40662371 Free PMC article. No abstract available.
Abstract
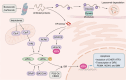
Apoptosis is a form of programmed cell death that is mediated by intrinsic and extrinsic pathways. Dysregulation of and resistance to cell death are hallmarks of cancer. For over three decades, the development of therapies to promote treatment of cancer by inducing various cell death modalities, including apoptosis, has been a main goal of clinical oncology. Apoptosis pathways also interact with other signaling mechanisms, such as the p53 signaling pathway and the integrated stress response (ISR) pathway. In addition to agents directly targeting the intrinsic and extrinsic pathway components, anticancer drugs that target the p53 and ISR signaling pathways are actively being developed. In this Review, we discuss selected and promising anticancer therapies in various stages of development, including drug targets, mechanisms, and resistance to related treatments, focusing especially on B cell lymphoma 2 (BCL-2) inhibitors, TRAIL analogues, DR5 antibodies, and strategies that target p53, mutant p53, and the ISR.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

