Tumor Vessel Normalization via PFKFB3 Inhibition Alleviates Hypoxia and Increases Tumor Necrosis in Rectal Cancer upon Radiotherapy
- PMID: 39007350
- PMCID: PMC11310748
- DOI: 10.1158/2767-9764.CRC-24-0077
Tumor Vessel Normalization via PFKFB3 Inhibition Alleviates Hypoxia and Increases Tumor Necrosis in Rectal Cancer upon Radiotherapy
Abstract
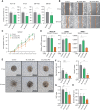
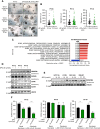
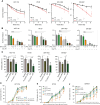
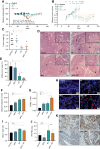
Treatment of patients with locally advanced rectal cancer (RC) is based on neoadjuvant chemoradiotherapy followed by surgery. In order to reduce the development of therapy resistance, it is necessary to further improve previous treatment approaches. Recent in vivo experimental studies suggested that the reduction of tumor hypoxia by tumor vessel normalization (TVN), through the inhibition of the glycolytic activator PFKFB3, could significantly improve tumor response to therapy. We have evaluated in vitro and in vivo the effects of the PFKFB3 inhibitor 2E-3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) on cell survival, clonogenicity, migration, invasion, and metabolism using colorectal cancer cells, patient-derived tumor organoid (PDO), and xenograft (PDX). 3PO treatment of colorectal cancer cells increased radiation-induced cell death and reduced cancer cell invasion. Moreover, gene set enrichment analysis shows that 3PO is able to alter the metabolic status of PDOs toward oxidative phosphorylation. Additionally, in vivo neoadjuvant treatment with 3PO induced TVN, alleviated tumor hypoxia, and increased tumor necrosis. Our results support PFKFB3 inhibition as a possible future neoadjuvant addition for patients with RC.
Significance: Novel therapies to better treat colorectal cancer are necessary to improve patient outcomes. Therefore, in this study, we evaluated the combination of a metabolic inhibitor (3PO) and standard radiotherapy in different experimental settings. We have observed that the addition of 3PO increased radiation effects, ultimately improving tumor cell response to therapy.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
N.B. Paul reports grants from the German Research Foundation during the conduct of the study and outside the submitted work. No disclosures were reported by the other authors.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688–96. - PubMed
-
- Martens MH, Maas M, Heijnen LA, Lambregts DMJ, Leijtens JWA, Stassen LPS, et al. Long-term outcome of an organ preservation program after neoadjuvant treatment for rectal cancer. J Natl Cancer Inst 2016;108:djw171. - PubMed
-
- van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 2018;391:2537–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

