Mitochondrial bioenergetics are not associated with myofibrillar protein synthesis rates
- PMID: 39007407
- PMCID: PMC11446679
- DOI: 10.1002/jcsm.13532
Mitochondrial bioenergetics are not associated with myofibrillar protein synthesis rates
Abstract
Background: Mitochondria represent key organelles influencing cellular homeostasis and have been implicated in the signalling events regulating protein synthesis.
Methods: We examined whether mitochondrial bioenergetics (oxidative phosphorylation and reactive oxygen species (H2O2) emission, ROS) measured in vitro in permeabilized muscle fibres represent regulatory factors for integrated daily muscle protein synthesis rates and skeletal muscle mass changes across the spectrum of physical activity, including free-living and bed-rest conditions: n = 19 healthy, young men (26 ± 4 years, 23.4 ± 3.3 kg/m2) and following 12 weeks of resistance-type exercise training: n = 10 healthy older men (70 ± 3 years, 25.2 ± 2.1 kg/m2). Additionally, we evaluated the direct relationship between attenuated mitochondrial ROS emission and integrated daily myofibrillar and sarcoplasmic protein synthesis rates in genetically modified mice (mitochondrial-targeted catalase, MCAT).
Results: Neither oxidative phosphorylation nor H2O2 emission were associated with muscle protein synthesis rates in healthy young men under free-living conditions or following 1 week of bed rest (both P > 0.05). Greater increases in GSSG concentration were associated with greater skeletal muscle mass loss following bed rest (r = -0.49, P < 0.05). In older men, only submaximal mitochondrial oxidative phosphorylation (corrected for mitochondrial content) was positively associated with myofibrillar protein synthesis rates during exercise training (r = 0.72, P < 0.05). However, changes in oxidative phosphorylation and H2O2 emission were not associated with changes in skeletal muscle mass following training (both P > 0.05). Additionally, MCAT mice displayed no differences in myofibrillar (2.62 ± 0.22 vs. 2.75 ± 0.15%/day) and sarcoplasmic (3.68 ± 0.35 vs. 3.54 ± 0.35%/day) protein synthesis rates when compared with wild-type mice (both P > 0.05).
Conclusions: Mitochondrial oxidative phosphorylation and reactive oxygen emission do not seem to represent key factors regulating muscle protein synthesis or muscle mass regulation across the spectrum of physical activity.
Keywords: Aging; Muscle protein synthesis; Physical inactivity; Reactive oxygen species; Skeletal muscle.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing interests.
Figures




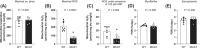

References
-
- Muller FL, Song W, Liu Y, Chaudhuri A, Pieke‐Dahl S, Strong R, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age‐dependent skeletal muscle atrophy. Free Radical Biol Med 2006;40:1993–2004. - PubMed
-
- St‐Jean‐Pelletier F, Pion CH, Leduc‐Gaudet J‐P, Sgarioto N, Zovilé I, Barbat‐Artigas S, et al. The impact of ageing, physical activity, and pre‐frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J Cachexia Sarcopenia Muscle 2017;8:213–228. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

