Temporary omission of oral anticoagulation in atrial fibrillation patients undergoing percutaneous coronary intervention: rationale and design of the WOEST-3 randomised trial
- PMID: 39007830
- PMCID: PMC11228535
- DOI: 10.4244/EIJ-D-24-00100
Temporary omission of oral anticoagulation in atrial fibrillation patients undergoing percutaneous coronary intervention: rationale and design of the WOEST-3 randomised trial
Abstract
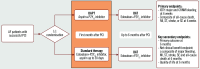
The optimal antithrombotic management of atrial fibrillation (AF) patients who require oral anticoagulation (OAC) undergoing percutaneous coronary intervention (PCI) remains unclear. Current guidelines recommend dual antithrombotic therapy (DAT; OAC plus P2Y12 inhibitor - preferably clopidogrel) after a short course of triple antithrombotic therapy (TAT; DAT plus aspirin). Although DAT reduces bleeding risk compared to TAT, this is counterbalanced by an increase in ischaemic events. Aspirin provides early ischaemic benefit, but TAT is associated with an increased haemorrhagic burden; therefore, we propose a 30-day dual antiplatelet therapy (DAPT; aspirin plus P2Y12 inhibitor) strategy post-PCI, temporarily omitting OAC. The study aims to compare bleeding and ischaemic risk between a 30-day DAPT strategy following PCI and a guideline-directed therapy in AF patients requiring OAC. WOEST-3 (ClinicalTrials.gov: NCT04436978) is an investigator-initiated, international, open-label, randomised controlled trial (RCT). AF patients requiring OAC who have undergone successful PCI will be randomised within 72 hours after PCI to guideline-directed therapy (edoxaban plus P2Y12 inhibitor plus limited duration of aspirin) or a 30-day DAPT strategy (P2Y12 inhibitor plus aspirin, immediately discontinuing OAC) followed by DAT (edoxaban plus P2Y12 inhibitor). With a sample size of 2,000 patients, this trial is powered to assess both superiority for major or clinically relevant non-major bleeding and non-inferiority for a composite of all-cause death, myocardial infarction, stroke, systemic embolism or stent thrombosis. In summary, the WOEST-3 trial is the first RCT temporarily omitting OAC in AF patients, comparing a 30-day DAPT strategy with guideline-directed therapy post-PCI to reduce bleeding events without hampering efficacy.
Conflict of interest statement
The authors have no specific conflicts of interest to declare with respect to this manuscript.
Figures
References
-
- Limbruno U, De Sensi, Cresti A, Picchi A, Lena F, De Caterina. Optimal Antithrombotic Treatment of Patients with Atrial Fibrillation Early after an Acute Coronary Syndrome-Triple Therapy, Dual Antithrombotic Therapy with an Anticoagulant… Or, Rather, Temporary Dual Antiplatelet Therapy? J Clin Med. 2020;9:2673. - PMC - PubMed
-
- Limbruno U, Goette A, De Caterina. Commentary: Temporarily omitting oral anticoagulants early after stenting for acute coronary syndromes patients with atrial fibrillation. Int J Cardiol. 2020;318:82–5. - PubMed
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder, Van Putte, Watkins CL ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. - PubMed
-
- Bang CN, Gislason GH, Greve AM, Bang CA, Lilja A, Torp-Pedersen C, Andersen PK, Køber L, Devereux RB, Wachtell K. New-onset atrial fibrillation is associated with cardiovascular events leading to death in a first time myocardial infarction population of 89,703 patients with long-term follow-up: a nationwide study. J Am Heart Assoc. 2014;3:e000382. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

