Durability of transcatheter aortic valve implantation
- PMID: 39007831
- PMCID: PMC11228542
- DOI: 10.4244/EIJ-D-23-01050
Durability of transcatheter aortic valve implantation
Abstract
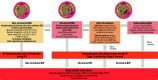
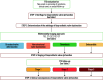
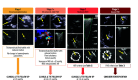
Transcatheter aortic valve implantation (TAVI) is now utilised as a less invasive alternative to surgical aortic valve replacement (SAVR) across the whole spectrum of surgical risk. Long-term durability of the bioprosthetic valves has become a key goal of TAVI as this procedure is now considered for younger and lower-risk populations. The purpose of this article is to present a state-of-the-art overview on the definition, aetiology, risk factors, mechanisms, diagnosis, clinical impact, and management of bioprosthetic valve dysfunction (BVD) and failure (BVF) following TAVI with a comparative perspective versus SAVR. Structural valve deterioration (SVD) is the main factor limiting the durability of the bioprosthetic valves used for TAVI or SAVR, but non-structural BVD, such as prosthesis-patient mismatch and paravalvular regurgitation, as well as valve thrombosis or endocarditis may also lead to BVF. The incidence of BVF related to SVD or other causes is low (<5%) at midterm (5- to 8-year) follow-up and compares favourably with that of SAVR. The long-term follow-up data of randomised trials conducted with the first generations of transcatheter heart valves also suggest similar valve durability in TAVI versus SAVR at 10 years, but these trials suffer from major survivorship bias, and the long-term durability of TAVI will need to be confirmed by the analysis of the low-risk TAVI versus SAVR trials at 10 years.
Conflict of interest statement
J. Ternacle has been a consultant for Abbott, Edwards Lifesciences, Pi-Cardia, Philips Healthcare, and GE HealthCare. M-A. Clavel has received funds from Edwards Lifesciences for computed tomography core laboratory analyses in the field of surgical bioprostheses; and research grants from Edwards Lifesciences, Medtronic, and Pi-Cardia, with no personal compensation. P. Pibarot has received funding from Edwards Lifesciences, Pi-Cardia, and Cardiac Success for echocardiography core laboratory analyses in the field of transcatheter valve therapies, and Medtronic for
Figures







References
-
- Mack MJ, Leon MB, Thourani VH, Pibarot P, Hahn RT, Genereux P, Kodali SK, Kapadia SR, Cohen DJ, Pocock SJ, Lu M, White R, Szerlip M, Ternacle J, Malaisrie SC, Herrmann HC, Szeto WY, Russo MJ, Babaliaros V, Smith CR, Blanke P, Webb JG, Makkar R PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N Engl J Med. 2023;389:1949–60. - PubMed
-
- Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, Bajwa T, Teirstein PS, Tchétché D, Huang J, Reardon MJ Evolut Low Risk Trial Investigators. 4-Year Outcomes of Patients With Aortic Stenosis in the Evolut Low Risk Trial. J Am Coll Cardiol. 2023;82:2163–5. - PubMed
-
- Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T, Lancellotti P, Sondergaard L, Ludman PF, Tamburino C, Piazza N, Hancock J, Mehilli J, Byrne RA, Baumbach A, Kappetein AP, Windecker S, Bax J, Haude M. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2017;38:3382–90. - PubMed
-
- VARC-3 WRITING Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, Finn MT, Kapadia S, Linke A, Mack MJ, Makkar R, Mehran R, Popma JJ, Reardon M, Rodes-Cabau J, Van Mieghem NM, Webb JG, Cohen DJ, Leon MB. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42:1825–57. - PubMed
-
- Pibarot P, Herrmann HC, Wu C, Hahn RT, Otto CM, Abbas AE, Chambers J, Dweck MR, Leipsic JA, Simonato M, Rogers T, Sathananthan J, Guerrero M, Ternacle J, Wijeysundera HC, Sondergaard L, Barbanti M, Salaun E, Généreux P, Kaneko T, Landes U, Wood DA, Deeb GM, Sellers SL, Lewis J, Madhavan M, Gillam L, Reardon M, Bleiziffer S, O’Gara PT, Rodés-Cabau J, Grayburn PA, Lancellotti P, Thourani VH, Bax JJ, Mack MJ, Leon MB Heart Valve Collaboratory. Standardized Definitions for Bioprosthetic Valve Dysfunction Following Aortic or Mitral Valve Replacement: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;80:545–61. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

