Determination of bisphosphonate properties in terms of bioavailability, bone affinity, and cytotoxicity
- PMID: 39007946
- PMCID: PMC11387443
- DOI: 10.1007/s43440-024-00624-2
Determination of bisphosphonate properties in terms of bioavailability, bone affinity, and cytotoxicity
Abstract
Background: The study aimed to evaluate the therapeutic potential of fourteen newly synthesized bisphosphonates by assessing their bioavailability, bone affinity, and cytotoxicity. These bisphosphonates included a series of aminomethylenebisphosphonates and standard compounds such as risedronate and tiludronate.
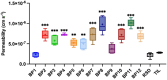
Methods: Drug permeability was determined using Parallel Artificial Membrane Permeability Assays (PAMPA), while bone affinity was assessed by sorption on hydroxyapatite. Bacterial cell response to the bisphosphonates was also examined using Lactobacillus paracasei cells as a model.
Results: Several tested compounds, including BP3 to BP8 and BP11, which feature substituents in the pyridine ring such as methyl groups, iodine, bromine, chlorine, or hydroxyl groups, demonstrated potentially more beneficial therapeutic properties than commercially used bisphosphonates. These compounds showed stronger bone affinity and higher gastrointestinal absorption with comparable or lower cytotoxic effects. Specifically, BP11 exhibited the highest bone affinity, while BP8 and BP11 showed the greatest permeability.
Conclusions: The findings suggest that BP3 BP8, and BP11 are promising candidates for further research. These results highlight the importance of comprehensively evaluating bisphosphonates' therapeutic properties to identify effective treatments for osteoporosis and other bone diseases.
Keywords: Artificial membrane permeability; Bisphosphonates; Cytotoxicity; Hydroxyapatite.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Comparative analysis of risedronate and its regioisomers synthesized via microwave-assisted method: bone affinity, cytotoxicity, permeability, and therapeutic potential.Pharmacol Rep. 2025 Apr;77(2):517-531. doi: 10.1007/s43440-025-00703-y. Epub 2025 Feb 10. Pharmacol Rep. 2025. PMID: 39928090
-
Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite.Bone. 2006 May;38(5):617-27. doi: 10.1016/j.bone.2005.05.003. Epub 2005 Jul 20. Bone. 2006. PMID: 16046206
-
[Pharmacokinetics of bisphosphonate.].Clin Calcium. 2016;26(11):1561-1570. Clin Calcium. 2016. PMID: 27777389 Japanese.
-
Bisphosphonates, Old Friends of Bones and New Trends in Clinics.J Med Chem. 2021 Feb 11;64(3):1260-1282. doi: 10.1021/acs.jmedchem.0c01292. Epub 2021 Feb 1. J Med Chem. 2021. PMID: 33522236 Review.
-
Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy.Osteoporos Int. 2008 Jun;19(6):733-59. doi: 10.1007/s00198-007-0540-8. Osteoporos Int. 2008. PMID: 18214569 Review.
Cited by
-
Injectable magnesium-bisphosphonate MOF-based bone adhesive prevents excessive fibrosis for osteoporotic fracture repair.Nat Commun. 2025 Jul 1;16(1):5679. doi: 10.1038/s41467-025-60853-8. Nat Commun. 2025. PMID: 40593608 Free PMC article.
References
-
- Clunie G, Keen RW. Osteoporosis. 2nd ed. Oxford: Oxford University Press; 2014.
-
- Ebetino FH, Rozé CN, McKenna CE, Barnett BL, Dunford JE, Russell RGG, et al. Molecular interactions of nitrogen-containing bisphosphonates within farnesyl diphosphate synthase. J Organomet Chem. 2005;690:2679–87. 10.1016/j.jorganchem.2005.03.005.10.1016/j.jorganchem.2005.03.005 - DOI
-
- Petneházy I, Jászay ZM, Töke L. Phosphite addition to carbonyl group and phosphoryl migration under phase transfer catalytic circumstances. Phosphorus Sulfur Silicon Relat Elem. 1996;109:421–4. 10.1080/10426509608545180.10.1080/10426509608545180 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
