Mitochondrial antioxidants abate SARS-COV-2 pathology in mice
- PMID: 39008677
- PMCID: PMC11287122
- DOI: 10.1073/pnas.2321972121
Mitochondrial antioxidants abate SARS-COV-2 pathology in mice
Abstract
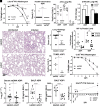
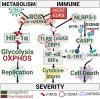
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection inhibits mitochondrial oxidative phosphorylation (OXPHOS) and elevates mitochondrial reactive oxygen species (ROS, mROS) which activates hypoxia-inducible factor-1alpha (HIF-1α), shifting metabolism toward glycolysis to drive viral biogenesis but also causing the release of mitochondrial DNA (mtDNA) and activation of innate immunity. To determine whether mitochondrially targeted antioxidants could mitigate these viral effects, we challenged mice expressing human angiotensin-converting enzyme 2 (ACE2) with SARS-CoV-2 and intervened using transgenic and pharmacological mitochondrially targeted catalytic antioxidants. Transgenic expression of mitochondrially targeted catalase (mCAT) or systemic treatment with EUK8 decreased weight loss, clinical severity, and circulating levels of mtDNA; as well as reduced lung levels of HIF-1α, viral proteins, and inflammatory cytokines. RNA-sequencing of infected lungs revealed that mCAT and Eukarion 8 (EUK8) up-regulated OXPHOS gene expression and down-regulated HIF-1α and its target genes as well as innate immune gene expression. These data demonstrate that SARS-CoV-2 pathology can be mitigated by catalytically reducing mROS, potentially providing a unique host-directed pharmacological therapy for COVID-19 which is not subject to viral mutational resistance.
Keywords: EUK8; SARS-CoV-2; antioxidant therapy; mCAT; mitochondria.
Conflict of interest statement
Competing interests statement:D.C.W. is on the scientific advisory boards of Pano Therapeutics, Inc. and Medical Excellent Capital, and published a manuscript with S.E. Schriner in 2022.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

