3D printed O2-generating scaffolds enhance osteoprogenitor- and type H vessel recruitment during bone healing
- PMID: 39009209
- PMCID: PMC11405102
- DOI: 10.1016/j.actbio.2024.07.011
3D printed O2-generating scaffolds enhance osteoprogenitor- and type H vessel recruitment during bone healing
Abstract
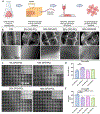
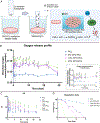
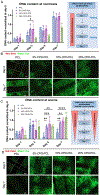
Oxygen (O2)-delivering tissue substitutes have shown tremendous potential for enhancing tissue regeneration, maturation, and healing. As O2 is both a metabolite and powerful signaling molecule, providing controlled delivery is crucial for optimizing its beneficial effects in the treatment of critical-sized injuries. Here, we report the design and fabrication of 3D-printed, biodegradable, O2-generating bone scaffold comprising calcium peroxide (CPO) that once hydrolytically activated, provides long-term generation of oxygen at a controlled, concentration-dependent manner, and polycaprolactone (PCL), a hydrophobic polymer that regulate the interaction of CPO with water, preventing burst release of O2 at early time points. When anoxic conditions were simulated in vitro, CPO-PCL scaffolds maintained the survival and proliferation of human adipose-derived stem/stromal cells (hASCs) relative to PCL-only controls. We assessed the in vivo osteogenic efficacy of hASC-seeded CPO-PCL scaffolds implanted in a non-healing critical-sized 4-mm calvarial defects in nude mice for 8 weeks. Even without exogenous osteoinductive factors, CPO-PCL scaffolds demonstrated increased new bone volume compared to PCL-only scaffolds as verified by both microcomputed tomography analysis and histological assessments. Lastly, we employed a quantitative 3D lightsheet microscopy platform to determine that O2-generating scaffolds had similar vascular volumes with slightly higher presence of CD31hiEmcnhi pro-osteogenic, type H vessels and increased number of Osterix+ skeletal progenitor cells relative to PCL-only scaffolds. In summary, 3D-printed O2 generating CPO-PCL scaffolds with tunable O2 release rates provide a facile, customizable strategy for effectively treating, craniofacial bone defects. STATEMENT OF SIGNIFICANCE: Oxygen(O2)-delivering bone substitutes show promise in defect repair applications by supplying O2 to the cells within or around the graft, improving cell survivability and enhancing bone matrix mineralization. A novel O2-generating bone scaffold has been 3D printed for the first-time which ensures patient and defect specificity. 3D printed calcium peroxide-polycaprolactone (CPO-PCL) bone scaffold provides uninterrupted O2 supply for 22 days allowing cell survival in deprived O2 and nutrient conditions. For the first time, O2-driven bone regenerative environment in mice calvaria has been captured by light-sheet imaging which illuminates abundance of Osterix+ cells, angiogenesis at a single cell resolution indicating active site of bone remodeling and growth in the presence of O2.
Keywords: Angiogenesis; Bone regeneration; Bone tissue engineering; Calcium peroxide; Oxygen.
Copyright © 2024 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

