Quantitative 3D electron microscopy characterization of mitochondrial structure, mitophagy, and organelle interactions in murine atrial fibrillation
- PMID: 39009246
- PMCID: PMC11381154
- DOI: 10.1016/j.jsb.2024.108110
Quantitative 3D electron microscopy characterization of mitochondrial structure, mitophagy, and organelle interactions in murine atrial fibrillation
Abstract
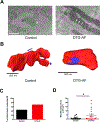
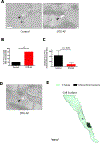
Atrial fibrillation (AF) is the most common clinical arrhythmia, however there is limited understanding of its pathophysiology including the cellular and ultrastructural changes rendered by the irregular rhythm, which limits pharmacological therapy development. Prior work has demonstrated the importance of reactive oxygen species (ROS) and mitochondrial dysfunction in the development of AF. Mitochondrial structure, interactions with other organelles such as sarcoplasmic reticulum (SR) and T-tubules (TT), and degradation of dysfunctional mitochondria via mitophagy are important processes to understand ultrastructural changes due to AF. However, most analysis of mitochondrial structure and interactome in AF has been limited to two-dimensional (2D) modalities such as transmission electron microscopy (EM), which does not fully visualize the morphological evolution of the mitochondria during mitophagy. Herein, we utilize focused ion beam-scanning electron microscopy (FIB-SEM) and perform reconstruction of three-dimensional (3D) EM from murine left atrial samples and measure the interactions of mitochondria with SR and TT. We developed a novel 3D quantitative analysis of FIB-SEM in a murine model of AF to quantify mitophagy stage, mitophagosome size in cardiomyocytes, and mitochondrial structural remodeling when compared with control mice. We show that in our murine model of spontaneous and continuous AF due to persistent late sodium current, left atrial cardiomyocytes have heterogenous mitochondria, with a significant number which are enlarged with increased elongation and structural complexity. Mitophagosomes in AF cardiomyocytes are located at Z-lines where they neighbor large, elongated mitochondria. Mitochondria in AF cardiomyocytes show increased organelle interaction, with 5X greater contact area with SR and are 4X as likely to interact with TT when compared to control. We show that mitophagy in AF cardiomyocytes involves 2.5X larger mitophagosomes that carry increased organelle contents. In conclusion, when oxidative stress overcomes compensatory mechanisms, mitophagy in AF faces a challenge of degrading bulky complex mitochondria, which may result in increased SR and TT contacts, perhaps allowing for mitochondrial Ca2+ maintenance and antioxidant production.
Keywords: 3D structure; Atrial fibrillation; Electron microscopy; Mitochondria; Mitophagy.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Avula UMR, Dridi H, Chen B-X, Yuan Q, Katchman AN, Reiken SR, Desai AD, Parsons S, Baksh H, Ma E, Dasrat P, Ji R, Lin Y, Sison C, Lederer WJ, Joca HC, Ward CW, Greiser M, Marks AR, Marx SO, Wan EY, 2021. Attenuating persistent sodium current–induced atrial myopathy and fibrillation by preventing mitochondrial oxidative stress. JCI Insight 6. - PMC - PubMed
-
- Chaanine AH, Joyce LD, Stulak JM, Maltais S, Joyce DL, Dearani JA, Klaus K, Nair KS, Hajjar RJ, Redfield MM, 2019. Mitochondrial Morphology, Dynamics, and Function in Human Pressure Overload or Ischemic Heart Disease With Preserved or Reduced Ejection Fraction. Circulation. Heart failure 12, e005131. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

