The molecular and cellular hematopoietic stem cell specification niche
- PMID: 39009276
- PMCID: PMC11338702
- DOI: 10.1016/j.exphem.2024.104280
The molecular and cellular hematopoietic stem cell specification niche
Abstract
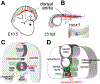
Hematopoietic stem cells (HSCs) are a population of tissue-specific stem cells that reside in the bone marrow of adult mammals, where they self-renew and continuously regenerate the adult hematopoietic lineages over the life of the individual. Prominence as a stem cell model and clinical usefulness have driven interest in understanding the physiologic processes that lead to the specification of HSCs during embryonic development. High-efficiency directed differentiation of HSCs by the instruction of defined progenitor cells using sequentially defined instructive molecules and conditions remains impossible, indicating that comprehensive knowledge of the complete set of precursor intermediate identities and required inductive inputs remains incompletely understood. Recently, interest in the molecular and cellular microenvironment where HSCs are specified from endothelial precursors-the "specification niche"-has increased. Here we review recent progress in understanding these niche spaces across vertebrate phyla, as well as how a better characterization of the origin and molecular phenotypes of the niche cell populations has helped inform and complicate previous understanding of signaling required for HSC emergence and maturation.
Copyright © 2024 International Society for Experimental Hematology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Disclosure The authors do not have any conflicts of interest to declare in relation to this work.
Figures
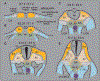
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

