Assembling a Coculture System to Prepare Highly Pure Induced Pluripotent Stem Cell-Derived Neurons at Late Maturation Stages
- PMID: 39009447
- PMCID: PMC11289586
- DOI: 10.1523/ENEURO.0165-24.2024
Assembling a Coculture System to Prepare Highly Pure Induced Pluripotent Stem Cell-Derived Neurons at Late Maturation Stages
Abstract
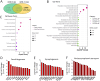
Generation of human induced pluripotent stem cell (hiPSC)-derived motor neurons (MNs) offers an unprecedented approach to modeling movement disorders such as dystonia and amyotrophic lateral sclerosis. However, achieving survival poses a significant challenge when culturing induced MNs, especially when aiming to reach late maturation stages. Utilizing hiPSC-derived motor neurons and primary mouse astrocytes, we assembled two types of coculture systems: direct coculturing of neurons with astrocytes and indirect coculture using culture inserts that physically separate neurons and astrocytes. Both systems significantly enhance neuron survival. Compared with these two systems, no significant differences in neurodevelopment, maturation, and survival within 3 weeks, allowing to prepare neurons at maturation stages. Using the indirect coculture system, we obtained highly pure MNs at the late mature stage from hiPSCs. Transcriptomic studies of hiPSC-derived MNs showed a typical neurodevelopmental switch in gene expression from the early immature stage to late maturation stages. Mature genes associated with neurodevelopment and synaptogenesis are highly enriched in MNs at late stages, demonstrating that these neurons achieve maturation. This study introduces a novel tool for the preparation of highly pure hiPSC-derived neurons, enabling the determination of neurological disease pathogenesis in neurons at late disease onset stages through biochemical approaches, which typically necessitate highly pure neurons. This advancement is particularly significant in modeling age-related neurodegeneration.
Keywords: coculture; human induced pluripotent stem cells (hiPSCs); motor neurons (MNs); neurodevelopment; synaptogenesis; transcriptomics.
Copyright © 2024 Akter et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Alves CJ, Dariolli R, Jorge FM, Monteiro MR, Maximino JR, Martins RS, Strauss BE, Krieger JE, Callegaro D, Chadi G (2015) Gene expression profiling for human iPS-derived motor neurons from sporadic ALS patients reveals a strong association between mitochondrial functions and neurodegeneration. Front Cell Neurosci 9:289. 10.3389/fncel.2015.00289 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
