Early-Life Resource Scarcity in Mice Does Not Alter Adult Corticosterone or Preovulatory Luteinizing Hormone Surge Responses to Acute Psychosocial Stress
- PMID: 39009448
- PMCID: PMC11287788
- DOI: 10.1523/ENEURO.0125-24.2024
Early-Life Resource Scarcity in Mice Does Not Alter Adult Corticosterone or Preovulatory Luteinizing Hormone Surge Responses to Acute Psychosocial Stress
Abstract
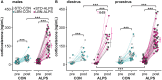
Early-life stressors can affect reproductive development and change responses to adult stress. We tested if resource scarcity in the form of limited bedding and nesting (LBN) from postnatal days (PND) 4 to 11 delayed sexual maturation in male and female mice and/or altered the response to an acute, layered, psychosocial stress (ALPS) in adulthood. Contrary to the hypotheses, age and mass at puberty were unaffected by the present application of LBN. Under basal conditions and after ALPS, corticosterone concentrations in males, diestrous females, and proestrous females reared in standard (STD) or LBN environments were similar. ALPS disrupts the luteinizing hormone (LH) surge in most mice when applied on the morning of proestrus; this effect was not changed by resource scarcity. In this study, the paucity of effects in the offspring may relate to a milder response of CBA dams to the paradigm. While LBN dams exited the nest more often and their offspring were smaller than STD-reared offspring on PND11, dam corticosterone concentrations were similar on PND11. To test if ALPS disrupts the LH surge by blunting the increase in excitatory GABAergic input to gonadotropin-releasing hormone (GnRH) neurons on the afternoon of proestrus, we conducted whole-cell voltage-clamp recordings. The frequency of GABAergic postsynaptic currents in GnRH neurons was not altered by LBN, ALPS, or their interaction. It remains possible that ALPS acts at afferents of GnRH neurons, changes response of GnRH neurons to input, and/or alters pituitary responsiveness to GnRH and that a more pronounced resource scarcity would affect the parameters studied.
Keywords: GABA; GnRH; LH; early-life stress; psychosocial stress; reproduction.
Copyright © 2024 Gibson and Moenter.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






References
-
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. 10.18637/jss.v067.i01 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
