Beta activity in human anterior cingulate cortex mediates reward biases
- PMID: 39009561
- PMCID: PMC11250824
- DOI: 10.1038/s41467-024-49600-7
Beta activity in human anterior cingulate cortex mediates reward biases
Erratum in
-
Author Correction: Beta activity in human anterior cingulate cortex mediates reward biases.Nat Commun. 2025 Jun 25;16(1):5397. doi: 10.1038/s41467-025-61277-0. Nat Commun. 2025. PMID: 40562769 Free PMC article. No abstract available.
Abstract
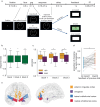
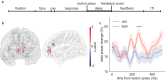
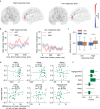
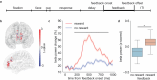
The rewards that we get from our choices and actions can have a major influence on our future behavior. Understanding how reward biasing of behavior is implemented in the brain is important for many reasons, including the fact that diminution in reward biasing is a hallmark of clinical depression. We hypothesized that reward biasing is mediated by the anterior cingulate cortex (ACC), a cortical hub region associated with the integration of reward and executive control and with the etiology of depression. To test this hypothesis, we recorded neural activity during a biased judgment task in patients undergoing intracranial monitoring for either epilepsy or major depressive disorder. We found that beta (12-30 Hz) oscillations in the ACC predicted both associated reward and the size of the choice bias, and also tracked reward receipt, thereby predicting bias on future trials. We found reduced magnitude of bias in depressed patients, in whom the beta-specific effects were correspondingly reduced. Our findings suggest that ACC beta oscillations may orchestrate the learning of reward information to guide adaptive choice, and, more broadly, suggest a potential biomarker for anhedonia and point to future development of interventions to enhance reward impact for therapeutic benefit.
© 2024. The Author(s).
Conflict of interest statement
S.A.S. has consulting agreements with Boston Scientific, NeuroPace, Abbott, and Zimmer Biomet. W.K.G. receives royalties from Nview, LLC and OCDscales, LLC. S.J.M. has served as a consultant to the following companies: Almatica Pharma, Biohaven, BioXcel Therapeutics, Boehringer-Ingelheim, Brii Biosciences, Clexio Biosciences, COMPASS Pathways, Delix Therapeutics, Douglas Pharmaceuticals, Eleusis, Engrail Therapeutics, Freedom Biosciences, Janssen, Liva Nova, Levo Therapeutics, Merck, Neumora, Neurocrine, Perception Neurosciences, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, Seelos Therapeutics, Signant Health, Sunovion, Xenon Pharmaceuticals, and XW Pharma. S.J.M. has received research support from Boehringer-Ingelheim, Engrail Therapeutics, Merck, Neurocrine, and Sage Therapeutics. N.P. is a consultant for Abbott Laboratories and Sensoria Therapeutics. The remaining authors declare no competing interests.
Figures

References
-
- Howard-Jones, P. A. & Jay, T. Reward, learning and games. Curr. Opin. Behav. Sci.10, 65–72 (2016). - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

