Investigating grey matter volumetric trajectories through the lifespan at the individual level
- PMID: 39009591
- PMCID: PMC11251262
- DOI: 10.1038/s41467-024-50305-0
Investigating grey matter volumetric trajectories through the lifespan at the individual level
Abstract
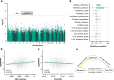
Adolescents exhibit remarkable heterogeneity in the structural architecture of brain development. However, due to limited large-scale longitudinal neuroimaging studies, existing research has largely focused on population averages, and the neurobiological basis underlying individual heterogeneity remains poorly understood. Here we identify, using the IMAGEN adolescent cohort followed up over 9 years (14-23 y), three groups of adolescents characterized by distinct developmental patterns of whole-brain gray matter volume (GMV). Group 1 show continuously decreasing GMV associated with higher neurocognitive performances than the other two groups during adolescence. Group 2 exhibit a slower rate of GMV decrease and lower neurocognitive performances compared with Group 1, which was associated with epigenetic differences and greater environmental burden. Group 3 show increasing GMV and lower baseline neurocognitive performances due to a genetic variation. Using the UK Biobank, we show these differences may be attenuated in mid-to-late adulthood. Our study reveals clusters of adolescent neurodevelopment based on GMV and the potential long-term impact.
© 2024. The Author(s).
Conflict of interest statement
Dr Banaschewski served in an advisory or consultancy role for Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH, Shire. He received conference support or speaker’s fee by Lilly, Medice, Novartis and Shire. He has been involved in clinical trials conducted by Shire & Viforpharma. He received royalties from Hogrefe, Kohlhammer, CIP Medien, Oxford University Press. The present work is unrelated to the above grants and relationships. Dr Barker receives honoraria for teaching from GE Healthcare. Dr Poustka served in an advisory or consultancy role for Roche and Viforpharm and received speaker’s fee by Shire. She received royalties from Hogrefe, Kohlhammer and Schattauer. The present work is unrelated to the above grants and relationships. The other authors declare no competing interests.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

