Efficient and reproducible generation of human iPSC-derived cardiomyocytes and cardiac organoids in stirred suspension systems
- PMID: 39009604
- PMCID: PMC11251028
- DOI: 10.1038/s41467-024-50224-0
Efficient and reproducible generation of human iPSC-derived cardiomyocytes and cardiac organoids in stirred suspension systems
Abstract
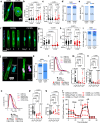
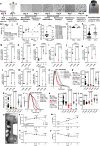
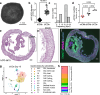
Human iPSC-derived cardiomyocytes (hiPSC-CMs) have proven invaluable for cardiac disease modeling and regeneration. Challenges with quality, inter-batch consistency, cryopreservation and scale remain, reducing experimental reproducibility and clinical translation. Here, we report a robust stirred suspension cardiac differentiation protocol, and we perform extensive morphological and functional characterization of the resulting bioreactor-differentiated iPSC-CMs (bCMs). Across multiple different iPSC lines, the protocol produces 1.2E6/mL bCMs with ~94% purity. bCMs have high viability after cryo-recovery (>90%) and predominantly ventricular identity. Compared to standard monolayer-differentiated CMs, bCMs are more reproducible across batches and have more mature functional properties. The protocol also works with magnetically stirred spinner flasks, which are more economical and scalable than bioreactors. Minor protocol modifications generate cardiac organoids fully in suspension culture. These reproducible, scalable, and resource-efficient approaches to generate iPSC-CMs and organoids will expand their applications, and our benchmark data will enable comparison to cells produced by other cardiac differentiation protocols.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
Efficient and reproducible generation of human iPSC-derived cardiomyocytes using a stirred bioreactor.bioRxiv [Preprint]. 2024 Feb 28:2024.02.24.581789. doi: 10.1101/2024.02.24.581789. bioRxiv. 2024. Update in: Nat Commun. 2024 Jul 15;15(1):5929. doi: 10.1038/s41467-024-50224-0. PMID: 38464269 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

