Fabrication, characterization and numerical validation of a novel thin-wall hydrogel vessel model for cardiovascular research based on a patient-specific stenotic carotid artery bifurcation
- PMID: 39009618
- PMCID: PMC11251049
- DOI: 10.1038/s41598-024-66777-5
Fabrication, characterization and numerical validation of a novel thin-wall hydrogel vessel model for cardiovascular research based on a patient-specific stenotic carotid artery bifurcation
Abstract
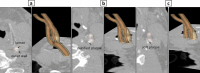
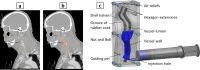
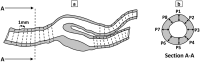
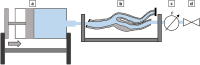
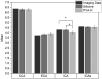
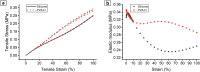
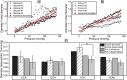
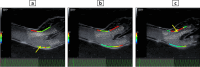
In vitro vascular models, primarily made of silicone, have been utilized for decades for studying hemodynamics and supporting the development of implants for catheter-based treatments of diseases such as stenoses and aneurysms. Hydrogels have emerged as prominent materials in tissue-engineering applications, offering distinct advantages over silicone models for fabricating vascular models owing to their viscoelasticity, low friction, and tunable mechanical properties. Our study evaluated the feasibility of fabricating thin-wall, anatomical vessel models made of polyvinyl alcohol hydrogel (PVA-H) based on a patient-specific carotid artery bifurcation using a combination of 3D printing and molding technologies. The model's geometry, elastic modulus, volumetric compliance, and diameter distensibility were characterized experimentally and numerically simulated. Moreover, a comparison with silicone models with the same anatomy was performed. A PVA-H vessel model was integrated into a mock circulatory loop for a preliminary ultrasound-based assessment of fluid dynamics. The vascular model's geometry was successfully replicated, and the elastic moduli amounted to 0.31 ± 0.007 MPa and 0.29 ± 0.007 MPa for PVA-H and silicone, respectively. Both materials exhibited nearly identical volumetric compliance (0.346 and 0.342% mmHg-1), which was higher compared to numerical simulation (0.248 and 0.290% mmHg-1). The diameter distensibility ranged from 0.09 to 0.20% mmHg-1 in the experiments and between 0.10 and 0.18% mmHg-1 in the numerical model at different positions along the vessel model, highlighting the influence of vessel geometry on local deformation. In conclusion, our study presents a method and provides insights into the manufacturing and mechanical characterization of hydrogel-based thin-wall vessel models, potentially allowing for a combination of fluid dynamics and tissue engineering studies in future cardio- and neurovascular research.
Keywords: Cardiovascular engineering; Fluid dynamics; Fluid–structure interaction (FSI); Hydrogel; In vitro; Numerical simulation; Ultrasound; Vessel compliance; Vessel model.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Development of an Experimental and Digital Cardiovascular Arterial Model for Transient Hemodynamic and Postural Change Studies: "A Preliminary Framework Analysis".Cardiovasc Eng Technol. 2018 Mar;9(1):1-31. doi: 10.1007/s13239-017-0332-z. Epub 2017 Nov 9. Cardiovasc Eng Technol. 2018. PMID: 29124548
-
A simulation environment for validating ultrasonic blood flow and vessel wall imaging based on fluid-structure interaction simulations: ultrasonic assessment of arterial distension and wall shear rate.Med Phys. 2010 Aug;37(8):4318-30. doi: 10.1118/1.3462592. Med Phys. 2010. PMID: 20879592
-
Walled vessel-mimicking phantom for ultrasound imaging using 3D printing with a water-soluble filament: design principle, fluid-structure interaction (FSI) simulation, and experimental validation.Phys Med Biol. 2020 Apr 27;65(8):085006. doi: 10.1088/1361-6560/ab7abf. Phys Med Biol. 2020. PMID: 32106096
-
Review: Mechanical Characterization of Carotid Arteries and Atherosclerotic Plaques.IEEE Trans Ultrason Ferroelectr Freq Control. 2016 Oct;63(10):1613-1623. doi: 10.1109/TUFFC.2016.2572260. Epub 2016 May 26. IEEE Trans Ultrason Ferroelectr Freq Control. 2016. PMID: 27249826 Review.
-
A Systematic Review for the Design of In Vitro Flow Studies of the Carotid Artery Bifurcation.Cardiovasc Eng Technol. 2020 Apr;11(2):111-127. doi: 10.1007/s13239-019-00448-9. Epub 2019 Dec 10. Cardiovasc Eng Technol. 2020. PMID: 31823191 Free PMC article.
References
-
- Geoghegan PH, Buchmann NA, Spence CJT, Moore S, Jermy M. Fabrication of rigid and flexible refractive-index-matched flow phantoms for flow visualisation and optical flow measurements. Exp. Fluids. 2012;52:1331–1347. doi: 10.1007/s00348-011-1258-0. - DOI
-
- Oh-Park, M., Zampolini, M. & Bitterman, J. Stroke syndromes. In Braddom’s Rehabilitation Care. A Clinical Handbook. Braddom’s Rehabilitation Care (ed. Cifu, D. X. & Lew, H. L.) 315–320 (Elsevier, 2018).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

