Evaluating psychological distress associated with life events under the traumatic experience threshold in patients with major depressive and bipolar disorder
- PMID: 39009703
- PMCID: PMC11250807
- DOI: 10.1038/s41598-024-67101-x
Evaluating psychological distress associated with life events under the traumatic experience threshold in patients with major depressive and bipolar disorder
Abstract
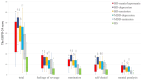
Patients with bipolar disorder (BD) and major depressive disorder (MDD) experience psychological distress associated with daily events that do not meet the threshold for traumatic experiences, referred to as event-related psychological distress (ERPD). Recently, we developed an assessment tool for ERPD, the ERPD-24. This tool considers four factors of ERPD: feelings of revenge, rumination, self-denial, and mental paralysis. We conducted a cross-sectional study between March 2021 and October 2022 to identify the differences and clinical features of ERPD among patients with MDD and BD and healthy subjects who did not experience traumatic events. Specifically, we assessed ERPD using the ERPD-24 and anxiety-related symptoms with the State-Trait Anxiety Inventory, Liebowitz Social Anxiety Scale, and anxious-depressive attack. Regarding the ERPD-24 scores among the groups, as the data did not rigorously follow the test of normality, the Kruskal-Wallis test was used to compare the differences among the groups, followed by the Dunn-Bonferroni adjusted post-hoc test. Non-remitted MDD patients and BD patients, regardless of remission/non-remission, presented more severe ERPD than healthy subjects. This study also demonstrated the relationships between all anxiety-related symptoms, including social phobia and anxious-depressive attack and ERPD, in both BD and MDD patients and in healthy subjects. In conclusion, patients with non-remitted MDD and with BD regardless of remission/non-remission experience severe ERPD related to anxiety-related symptoms.
© 2024. The Author(s).
Conflict of interest statement
T.H. received research fundings from Japan Research Foundation for Clinical Pharmacology, Mitsubishi Foundation, and READYFOR Corporation. M.I. received consultant fees from Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., Pfizer Japan Inc., Abbott Japan Co., Ltd. and Janssen Pharmaceutical K.K., and reports honoraria from Janssen Pharmaceutical K.K., Eli Lilly Japan K.K., Otsuka Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., GlaxoSmithKline K.K., Takeda Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., MSD K.K., Eisai Co. Ltd., Daiichi-Sankyo Co. Ltd., Novartis Pharma K.K., Teijin Ltd., Shionogi & Co., Ltd., Hisamitsu Pharmaceutical Co., Inc. and Asahi Kasei Corporation. M.N. reports honoraria from Takeda Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Daiichi-Sankyo Co. Ltd., Novartis Pharma K.K., and Lundbeck Japan K.K. A.S. reports honoraria from Takeda Pharmaceutical Co., Ltd., and Lundbeck Japan K.K. A.K. reports honoraria from Takeda Pharmaceutical Co., Ltd. The other authors declare that they have no conflict of interest.
Figures

References
-
- American Psychiatric Association. Diagnostic & Statistical Manual of Mental Disorders, 5th ed. (2013).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

