Engineering a nanoscale liposome-in-liposome for in situ biochemical synthesis and multi-stage release
- PMID: 39009794
- PMCID: PMC11446840
- DOI: 10.1038/s41557-024-01584-z
Engineering a nanoscale liposome-in-liposome for in situ biochemical synthesis and multi-stage release
Abstract
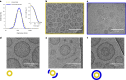
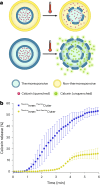
Soft-matter nanoscale assemblies such as liposomes and lipid nanoparticles have the potential to deliver and release multiple cargos in an externally stimulated and site-specific manner. Such assemblies are currently structurally simplistic, comprising spherical capsules or lipid clusters. Given that form and function are intertwined, this lack of architectural complexity restricts the development of more sophisticated properties. To address this, we have devised an engineering strategy combining microfluidics and conjugation chemistry to synthesize nanosized liposomes with two discrete compartments, one within another, which we term concentrisomes. We can control the composition of each bilayer and tune both particle size and the dimensions between inner and outer membranes. We can specify the identity of encapsulated cargo within each compartment, and the biophysical features of inner and outer bilayers, allowing us to imbue each bilayer with different stimuli-responsive properties. We use these particles for multi-stage release of two payloads at defined time points, and as attolitre reactors for triggered in situ biochemical synthesis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

