Clinical effects of teriparatide, abaloparatide, and romosozumab in postmenopausal osteoporosis
- PMID: 39009890
- PMCID: PMC11954689
- DOI: 10.1007/s00774-024-01536-0
Clinical effects of teriparatide, abaloparatide, and romosozumab in postmenopausal osteoporosis
Abstract
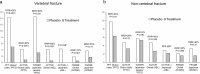
In the management of osteoporosis, anti-resorptive agents serve as a primary therapeutic approach. However, in cases where individuals exhibit an increased susceptibility to fractures, such as those characterized by severe low bone mass or a history of vertebral or hip fractures that markedly diminish life expectancy, the immediate reduction of fracture risk through the administration of osteoanabolic agents could be beneficial. Teriparatide, available in daily, once-weekly, or twice-weekly dosages, along with abaloparatide and romosozumab, constitutes a trio of such agents. Each of these medications is defined by unique characteristics, distinct efficacy profiles, and specific adverse effects. There is growing evidence to suggest that these agents have a superior effect on enhancing bone mineral density and reducing fracture incidence when compared to traditional bisphosphonate therapies. Nonetheless, their employment demands thorough consideration of clinical indications, which includes evaluating economic factors, the frequency of injections required, and the potential for adverse effects. The objective of this review is to consolidate the current evidence focusing primarily on the efficacy of these agents, with the goal of enhancing understanding and aiding in making more informed treatment decisions, particularly for those individuals who are at an elevated risk of fractures.
Keywords: Abaloparatide; Osteoanabolic agent; Postmenopausal osteoporosis; Romosozumab; Teriparatide.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: KE and YE are affiliated with the Department of Sports Medical Biomechanics, Osaka University Graduate School of Medicine, which is supported by Asahi-Kasei. KE has received research grants from Asahi-Kasei, Eisai, Ono, and Teijin Pharma. KE has received lecture fee from Amgen, Asahi-Kasei, Astellas, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Ono, Pfizer, Taisho, and Teijin Pharma. KE has received consultant fee from Asahi-Kasei, Taisho, and Teijin Pharma. YE has received a research grant from Eli Lilly. KN has received a research grant from Astellas and supervises the Department of Sports Medical Biomechanics, Osaka University Graduate School of Medicine, which is supported by Asahi-Kasei. TN and SO declare that they have no conflicts of interest. Ethical approval: Since this manuscript is a review article, it is not subject to the ethical standards of the institutional and/or national research committees or to the 1964 Helsinki Declaration and its subsequent amendments or comparable ethical standards.
Figures
References
-
- Cummings SR, Cosman F, Lewiecki EM, Schousboe JT, Bauer DC et al (2017) Goal-Directed Treatment for Osteoporosis: A Progress Report From the ASBMR-NOF Working Group on Goal-Directed Treatment for Osteoporosis. J Bone Miner Res 32:3–10 - PubMed
-
- Compston JE, McClung MR, Leslie WD (2019) Osteoporosis Lancet 393:364–376 - PubMed
-
- Black DM, Eastell R, Adams AL (2020) Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates. Reply N Engl J Med 383:2189–2190 - PubMed
-
- Black DM, Bauer DC, Vittinghoff E, Lui LY, Grauer A, Marin F, Khosla S, de Papp A, Mitlak B, Cauley JA, McCulloch CE, Eastell R, Bouxsein ML (2020) Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol 8:672–682 - PubMed
-
- Diez-Perez A, Adachi JD, Agnusdei D, Bilezikian JP, Compston JE, Cummings SR, Eastell R, Eriksen EF, Gonzalez-Macias J, Liberman UA, Wahl DA, Seeman E, Kanis JA, Cooper C (2012) Treatment failure in osteoporosis. Osteoporos Int 23:2769–2774 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

