Efficacy of chemotherapy plus immune checkpoint inhibitors in patients with non-small cell lung cancer who have rare oncogenic driver mutations: a retrospective analysis
- PMID: 39009968
- PMCID: PMC11247748
- DOI: 10.1186/s12885-024-12554-6
Efficacy of chemotherapy plus immune checkpoint inhibitors in patients with non-small cell lung cancer who have rare oncogenic driver mutations: a retrospective analysis
Abstract
Background: Targeted therapy is now the standard of care in driver-oncogene-positive non-small cell lung cancer (NSCLC). Its initial clinical effects are remarkable. However, almost all patients experience treatment resistance to targeted therapy. Hence, chemotherapy is considered a subsequent treatment option. In patients with driver-oncogene-negative NSCLC, combined immune checkpoint inhibitors (ICIs) and chemotherapy as the first-line therapy has been found to be beneficial. However, the efficacy of ICI plus chemotherapy against driver-oncogene-positive NSCLC other than epidermal growth factor receptor mutation and anaplastic lymphoma kinase fusion is unclear.
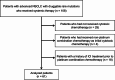
Methods: Using the hospital medical records, we retrospectively reviewed advanced or recurrent NSCLC patients who were treated with chemotherapy with or without ICIs at Aichi Cancer Center Hospital between January 2014 and January 2023. Patients with druggable rare mutations such as KRAS-G12C, MET exon 14 skipping, HER2 20 insertion, BRAF-V600E mutations, and ROS1 and RET rearrangements were analyzed.
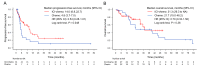
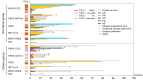
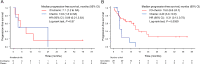
Results: In total, 61 patients were included in this analysis. ICI plus chemotherapy was administered in 36 patients (the ICI-chemo group) and chemotherapy in 25 patients (the chemo group). The median progression-free survival (PFS) rates were 14.0 months in the ICI-chemo group and 4.8 months in the chemo group (hazard ratio [HR] = 0.54, 95% confidence interval [CI] = 0.28-1.01). The median overall survival rates were 31.3 and 21.7 months in the ICI-chemo and chemo groups, respectively (HR = 0.70, 95% CI = 0.33-1.50). Multivariate Cox regression analysis of PFS revealed that HER2 exon 20 insertion mutation was significantly associated with a poorer PFS (HR: 2.39, 95% CI: 1.19-4.77, P = 0.014). Further, ICI-chemo treatment was significantly associated with a better PFS (HR: 0.48, 95% CI: 0.25-0.91, P = 0.025).
Conclusion: ICI plus chemotherapy improves treatment efficacy in rare driver-oncogene-positive NSCLC.
Keywords: Driver mutation; Immune checkpoint inhibitors; Immunotherapy; Non-small cell lung cancer.
© 2024. The Author(s).
Conflict of interest statement
T.Y. received personal fees from Chugai Pharmaceutical, Eli Lilly, Ono Pharmaceutical, Taiho Pharmaceutical, MSD, AstraZeneca, Daiichi Sankyo, Bristol-Meyers Squibb and Boehringer Ingelheim. J.S. received personal fees from Chugai Pharmaceutical, Bristol-Myers Squibb, Ono Pharmaceutical, MSD and AstraZeneca. R.M. received personal fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Chugai Pharmaceutical, Pfizer, Taiho Pharmaceutical, Ono Pharmaceutical, and MSD. Y.F. received grants from Chugai Pharmaceutical and personal fees from AstraZeneca, Ono Pharmaceutical, Novartis, Daiichi Sankyo, Yakult, and Otsuka Pharmaceutical. All remaining authors declare no conflicts of interest.
Figures
Similar articles
-
Clinical outcomes for immune checkpoint inhibitors plus chemotherapy in non-small-cell lung cancer patients with uncommon driver gene alterations.BMC Cancer. 2024 Aug 3;24(1):952. doi: 10.1186/s12885-024-12748-y. BMC Cancer. 2024. PMID: 39097705 Free PMC article.
-
Efficacy of first-line immune checkpoint inhibitors in patients with advanced NSCLC with KRAS, MET, FGFR, RET, BRAF, and HER2 alterations.Thorac Cancer. 2022 Jun;13(11):1703-1711. doi: 10.1111/1759-7714.14448. Epub 2022 May 2. Thorac Cancer. 2022. PMID: 35491960 Free PMC article.
-
Efficacy of immune checkpoint inhibitors plus platinum-based chemotherapy as 1st line treatment for patients with non-small cell lung cancer harboring HER2 mutations: Results from LC-SCRUM-Asia.Lung Cancer. 2024 Nov;197:107992. doi: 10.1016/j.lungcan.2024.107992. Epub 2024 Oct 13. Lung Cancer. 2024. PMID: 39423763
-
Efficacy of first-line immune checkpoint inhibitor and anti-angiogenic agent combination therapy for Kirsten rat sarcoma viral antigen-mutant advanced non-small-cell lung cancer: a systematic review and network meta-analysis.Thorac Cancer. 2024 Sep;15(25):1854-1862. doi: 10.1111/1759-7714.15413. Epub 2024 Jul 31. Thorac Cancer. 2024. PMID: 39086088 Free PMC article.
-
Immunotherapy for oncogenic-driven advanced non-small cell lung cancers: Is the time ripe for a change?Cancer Treat Rev. 2018 Dec;71:47-58. doi: 10.1016/j.ctrv.2018.10.006. Epub 2018 Oct 15. Cancer Treat Rev. 2018. PMID: 30359792 Review.
Cited by
-
Molecular principles underlying aggressive cancers.Signal Transduct Target Ther. 2025 Feb 17;10(1):42. doi: 10.1038/s41392-025-02129-7. Signal Transduct Target Ther. 2025. PMID: 39956859 Free PMC article. Review.
-
Understanding and overcoming multidrug resistance in cancer.Nat Rev Clin Oncol. 2025 Jul 29. doi: 10.1038/s41571-025-01059-1. Online ahead of print. Nat Rev Clin Oncol. 2025. PMID: 40731166 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

