Enteroviruses: epidemic potential, challenges and opportunities with vaccines
- PMID: 39010093
- PMCID: PMC11247760
- DOI: 10.1186/s12929-024-01058-x
Enteroviruses: epidemic potential, challenges and opportunities with vaccines
Abstract
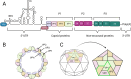
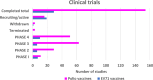
Enteroviruses (EVs) are the most prevalent viruses in humans. EVs can cause a range of acute symptoms, from mild common colds to severe systemic infections such as meningitis, myocarditis, and flaccid paralysis. They can also lead to chronic diseases such as cardiomyopathy. Although more than 280 human EV serotypes exist, only four serotypes have licenced vaccines. No antiviral drugs are available to treat EV infections, and global surveillance of EVs has not been effectively coordinated. Therefore, poliovirus still circulates, and there have been alarming epidemics of non-polio enteroviruses. Thus, there is a pressing need for coordinated preparedness efforts against EVs.This review provides a perspective on recent enterovirus outbreaks and global poliovirus eradication efforts with continuous vaccine development initiatives. It also provides insights into the challenges and opportunities in EV vaccine development. Given that traditional whole-virus vaccine technologies are not suitable for many clinically relevant EVs and considering the ongoing risk of enterovirus outbreaks and the potential for new emerging pathogenic strains, the need for new effective and adaptable enterovirus vaccines is emphasized.This review also explores the difficulties in translating promising vaccine candidates for clinical use and summarizes information from published literature and clinical trial databases focusing on existing enterovirus vaccines, ongoing clinical trials, the obstacles faced in vaccine development as well as the emergence of new vaccine technologies. Overall, this review contributes to the understanding of enterovirus vaccines, their role in public health, and their significance as a tool for future preparedness.
Keywords: EV outbreaks; EV surveillance; Enterovirus (EV); Vaccine development.
© 2024. The Author(s).
Conflict of interest statement
MJ, MFT and MMH have no conflicts of interest.
Figures

References
-
- International Committee on Taxonomy of Viruses: ICTV. Available at https://ictv.global/report/chapter/picornaviridae/picornaviridae/enterov....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

