Outcomes of patients with alcohol-associated hepatitis and acute kidney injury - Results from the HRS Harmony Consortium
- PMID: 39010302
- PMCID: PMC11349455
- DOI: 10.1111/apt.18159
Outcomes of patients with alcohol-associated hepatitis and acute kidney injury - Results from the HRS Harmony Consortium
Abstract
Background & aims: The development of acute kidney injury (AKI) in the setting of alcohol-associated hepatitis (AH) portends a poor prognosis. Whether the presence of AH itself drives worse outcomes in patients with cirrhosis and AKI is unknown.
Methods: Retrospective cohort study of 11 hospital networks of consecutive adult patients admitted in 2019 with cirrhosis and AKI. AKI phenotypes, clinical course, and outcomes were compared between AH and non-AH groups.
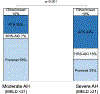
Results: A total of 2062 patients were included, of which 303 (15%) had AH, as defined by National Institute on Alcohol Abuse and Alcoholism (NIAAA) criteria. Patients with AH, compared to those without, were younger and had higher Model for End-stage Liver Disease-Sodium (MELD-Na) scores on admission. AKI phenotypes significantly differed between groups (p < 0.001) with acute tubular necrosis occurring more frequently in patients with AH. Patients with AH reached more severe peak AKI stage, required more renal replacement therapy, and had higher 90-day cumulative incidence of death (45% [95% CI: 39%-51%] vs. 38% [95% CI: 35%-40%], p = 0.026). Using no AH as reference, the unadjusted sHR for 90-day mortality was higher for AH (sHR: 1.24 [95% CI: 1.03-1.50], p = 0.024), but was not significant when adjusting for MELD-Na, age and sex. However, in patients with hepatorenal syndrome, AH was an independent predictor of 90-day mortality (sHR: 1.82 [95% CI: 1.16-2.86], p = 0.009).
Conclusions: Hospitalised patients with cirrhosis and AKI presenting with AH had higher 90-day mortality than those without AH, but this may have been driven by higher MELD-Na rather than AH itself. However, in patients with hepatorenal syndrome, AH was an independent predictor of mortality.
© 2024 John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
ASA reports consultant fees from Mallinckrodt Pharmaceuticals and Ocelot Bio. GC reports consulting fees from Ocelot Bio and Retro Biosciences. ESO reports consultant fees from Biovie. HMW reports consultant fees from Mallinckrodt Pharmaceuticals. KRR reports consultant fees from Mallinckrodt Pharmaceuticals. JMB reports consultant fees from Mallinckrodt Pharmaceuticals. The other authors have no conflict of interest to declare.
Figures
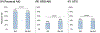


References
-
- Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306–33. - PubMed
-
- Singal AK, Arsalan A, Dunn W, Arab JP, Wong RJ, Kuo YF, et al. Alcohol-associated liver disease in the United States is associated with severe forms of disease among young, females and Hispanics. Aliment Pharmacol Ther. 2021;54:451–61. - PubMed
-
- Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–28. - PubMed
Publication types
MeSH terms
Grants and funding
- American Association for the Study of Liver Diseases Clinical, Translational, and Outcomes Research Award
- MGH Research Scholars Program
- L30 DK133959/DK/NIDDK NIH HHS/United States
- K23 AA031068/AA/NIAAA NIH HHS/United States
- K23 DK128567/DK/NIDDK NIH HHS/United States
- Merck
- Roche
- Gilead
- Bristol-Myers Squibb
- GlaxoSmithKline
- K23DK131278/NH/NIH HHS/United States
- K23 DK131278/DK/NIDDK NIH HHS/United States
- L30DK133959/NH/NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- P30 DK026743/DK/NIDDK NIH HHS/United States
- L30DK133959/National Institutes of Healt
- K23DK131278/National Institutes of Healt
- K23DK128567/NH/NIH HHS/United States
- K23AA031068/NH/NIH HHS/United States
- P30DK026743/UCSF Liver Center
- K23AA031068/National Institutes of Healt
- K23DK128567/National Institutes of Healt
LinkOut - more resources
Full Text Sources
