Role of silver nanoparticles and silver nanoclusters for the detection and removal of Hg(ii)
- PMID: 39010928
- PMCID: PMC11247438
- DOI: 10.1039/d4ra04182h
Role of silver nanoparticles and silver nanoclusters for the detection and removal of Hg(ii)
Abstract
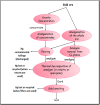
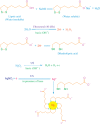
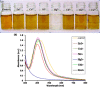
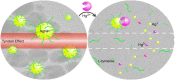
Silver metal, being a 3d transition metal in group 11 in the periodic table, is widely used in material science for its distinguished plasmonic properties. Nanoparticles (NPs) and nanoclusters (NCs) are widely used in sensing applications having a surface plasmon band and emissive properties, respectively. Mercury is one of the detrimental toxins and threats to various ecosystems. The distinction between nanoparticles and nanoclusters, the utility and toxicity of heavy metal mercury, fluorometric and colorimetric approaches to the recognition of mercury ions with NPs and NCs, the mechanism of detection, spot detection, and natural water sample analyses were illustrated in detail in this review article. Moreover, the sensing platform and analyte (Hg2+) fate were described for substantiating the mechanism. It was observed that NCs are mostly utilized for fluorometric approaches, while NPs are mostly employed for colorimetric approaches. Fluorometric detection is mainly quenching-based. However, sensing with enhancement was found in a few reports. Adulteration of other metals with silver particles often modifies the sensing platform.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures












References
-
- Chaudhry F. N. Malik M. F. J. Ecosyst. Ecography. 2017;7:1–3.
-
- Ahuja S. ACS Symp. Ser. 2020;1352:1–11.
-
- Mikes J. Siglova M. Cejkova A. Masak J. Jirku V. Water Sci. Technol. 2005;52:151–156. - PubMed
-
- Liu M. Zhang Q. Maavara T. Liu S. Wang X. Raymond P. A. Nat. Geosci. 2021;14:672–677.
Publication types
LinkOut - more resources
Full Text Sources

