Non-small cell lung cancer cells and concomitant cancer therapy induce a resistance-promoting phenotype of tumor-associated mesenchymal stem cells
- PMID: 39011489
- PMCID: PMC11246879
- DOI: 10.3389/fonc.2024.1406268
Non-small cell lung cancer cells and concomitant cancer therapy induce a resistance-promoting phenotype of tumor-associated mesenchymal stem cells
Abstract
Introduction: The tumor microenvironment gained attraction over the last decades as stromal cells significantly impact on tumor development, progression and metastasis, and immune evasion as well as on cancer therapy resistance. We previously reported that lung-resident mesenchymal stem cells (MSCs) were mobilized and activated in non-small cell lung cancer (NSCLC) progression and could even mediate radiation resistance in co-cultured NSCLC cells.
Methods: We investigated how MSCs were affected by NSCLC cells in combination with cancer (radiation) therapy in indirect co-cultures using tumor-conditioned medium and Transwells or direct three-dimensional NSCLC-MSC spheroid co-cultures in order to unravel the resistance-mediating action of tumor-associated MSCs.
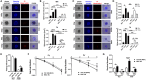
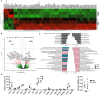
Results: Although no obvious phenotypic and functional alterations in MSCs following NSCLC co-culture could be observed, MSC senescence was induced following co-applied radiotherapy (RT). Global gene expression profiling, in combination with gene set enrichment analysis upon treatment, was used to confirm the senescent phenotype of irradiated MSC and to reveal relevant senescence-associated secretory phenotype (SASP) factors that could meditate NSCLC RT resistance. We identified senescent tumor-associated MSC-derived serine proteinase inhibitor (serpin) E1/PAI1 as potential SASP factor mediating NSCLC progression and RT resistance.
Discussion: Specified intra-tumor-stroma interactions and cell type-specific pro-tumorigenic functions could not only improve lung cancer classification but could even be used for a more precise profiling of individual patients, finally paving an additional way for the discovery of potential drug targets for NSCLC patients.
Keywords: NSCLC; SASP; adventitia; lung cancer; mesenchymal stem cells; radiotherapy; resistance; senescence.
Copyright © 2024 Sentek, Braun, Budeus and Klein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Zhao J, Xu L, Sun J, Song M, Wang L, Yuan S, et al. Global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019. BMJ Oncol. (2023) 2:e000049. doi: 10.1136/bmjonc-2023-000049 - DOI
-
- Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. (eds) Seer Cancer Statistics Review, 1975-2018. (2021), Based on November 2020 Seer Data Submission, Posted to the Seer Web Site.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

