Recent advances in the study of zika virus structure, drug targets, and inhibitors
- PMID: 39011504
- PMCID: PMC11246971
- DOI: 10.3389/fphar.2024.1418516
Recent advances in the study of zika virus structure, drug targets, and inhibitors
Abstract
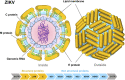
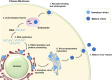
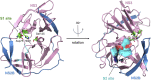
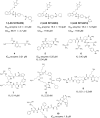
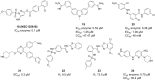
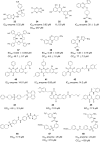
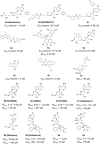
Zika Virus (ZIKV) is a positive-strand RNA virus that can lead to Guillain-Barré syndrome or encephalitis in some individuals and hence presents a serious public health risk. Since the first outbreak of ZIKV in Brazil in 2015, no effective clinical inhibitors have been developed, making the development of effective ZIKV drugs an urgent issue that needs to be addressed. ZIKV belongs to the Flaviviridae family, and its structure includes three structural proteins, namely, capsular (C), premembrane (prM), and envelope (E) proteins, as well as seven nonstructural proteins, namely, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. To provide a reference for the development of future ZIKV drugs, this paper reviews the structure of the ZIKV based on recent literature reports, analyzes the potential therapeutic targets of various proteins, and proposes feasible drug design strategies. Additionally, this paper reviews and classifies the latest research progress on several protease inhibitors, such as E protein inhibitors, NS2B-NS3 inhibitors, and NS5 inhibitors, so that researchers can quickly understand the current status of development and the interconnections among these inhibitors.
Keywords: MTase; RdRp; ZIKV; drug target; inhibitor.
Copyright © 2024 Feng.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
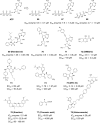
Figures
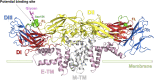




References
-
- Alcon S., Talarmin A., Debruyne M., Falconar A., Deubel V., Flamand M. (2002). Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40 (2), 376–381. 10.1128/JCM.40.02.376-381.2002 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

