Infection associated with CDK4/6 inhibitors: a pharmacovigilance analysis of the FDA adverse event reporting system database
- PMID: 39011505
- PMCID: PMC11247343
- DOI: 10.3389/fphar.2024.1371346
Infection associated with CDK4/6 inhibitors: a pharmacovigilance analysis of the FDA adverse event reporting system database
Abstract
Introduction: Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors are first-line treatments for hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer. With their increasing clinical use, infection-related adverse events (AEs) associated with CDK4/6 inhibitors have been widely reported in recent years. This study aimed to analyze the occurrence of infections associated with the CDK4/6 inhibitors (palbociclib, ribociclib and abemaciclib) based on the real-world data from the US Food and Drug Administration Adverse Event Reporting System (FAERS) database.
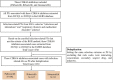
Methods: Data were extracted from the FAERS database between 2015Q1 and 2022Q3. The clinical characteristics of patients with primary suspected infection-related AEs were analyzed. A disproportionality analysis was performed to investigate the potential association between AEs and CDK4/6 inhibitors. The influencing factors were evaluated using Pearson's chi-square test.
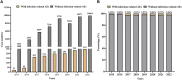
Results: Reports of infection-related AEs associated with ribociclib accounted for 8.58% of the total reports of AEs associated with ribociclib, followed by palbociclib (2.72%) and abemaciclib (1.24%). Ribociclib (67.65%) was associated with more serious outcome events than palbociclib (30%) or abemaciclib (48.08%). The sex and age were not associated with outcome severity. Disproportionality analysis showed that fourteen, sixteen and two infection-related preferred terms were detected for palbociclib, ribociclib and abemaciclib, respectively.
Conclusion: Infection-related AEs were highly associated with three CDK4/6 inhibitors, especially palbociclib and ribociclib, based on the real-world data from the FAERS database. However, further causality assessment is required.
Keywords: CDK4/6 inhibitor; FAERS; adverse events; infection; ribociclib.
Copyright © 2024 Chen, Tang, Song, Sun and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bas O., Erul E., Guven D. C., Aksoy S. (2022). Infectious complications of cyclin-dependent kinases 4 and 6 inhibitors in patients with hormone-receptor-positive metastatic breast cancer: a systematic review and meta-analysis. Support. Care. Cancer. 30 (11), 9071–9078. 10.1007/s00520-022-07320-y - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

