Post-translational modifications in prion diseases
- PMID: 39011540
- PMCID: PMC11247024
- DOI: 10.3389/fnmol.2024.1405415
Post-translational modifications in prion diseases
Abstract
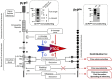
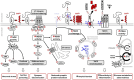
More than 650 reversible and irreversible post-translational modifications (PTMs) of proteins have been listed so far. Canonical PTMs of proteins consist of the covalent addition of functional or chemical groups on target backbone amino-acids or the cleavage of the protein itself, giving rise to modified proteins with specific properties in terms of stability, solubility, cell distribution, activity, or interactions with other biomolecules. PTMs of protein contribute to cell homeostatic processes, enabling basal cell functions, allowing the cell to respond and adapt to variations of its environment, and globally maintaining the constancy of the milieu interieur (the body's inner environment) to sustain human health. Abnormal protein PTMs are, however, associated with several disease states, such as cancers, metabolic disorders, or neurodegenerative diseases. Abnormal PTMs alter the functional properties of the protein or even cause a loss of protein function. One example of dramatic PTMs concerns the cellular prion protein (PrPC), a GPI-anchored signaling molecule at the plasma membrane, whose irreversible post-translational conformational conversion (PTCC) into pathogenic prions (PrPSc) provokes neurodegeneration. PrPC PTCC into PrPSc is an additional type of PTM that affects the tridimensional structure and physiological function of PrPC and generates a protein conformer with neurotoxic properties. PrPC PTCC into PrPSc in neurons is the first step of a deleterious sequence of events at the root of a group of neurodegenerative disorders affecting both humans (Creutzfeldt-Jakob diseases for the most representative diseases) and animals (scrapie in sheep, bovine spongiform encephalopathy in cow, and chronic wasting disease in elk and deer). There are currently no therapies to block PrPC PTCC into PrPSc and stop neurodegeneration in prion diseases. Here, we review known PrPC PTMs that influence PrPC conversion into PrPSc. We summarized how PrPC PTCC into PrPSc impacts the PrPC interactome at the plasma membrane and the downstream intracellular controlled protein effectors, whose abnormal activation or trafficking caused by altered PTMs promotes neurodegeneration. We discussed these effectors as candidate drug targets for prion diseases and possibly other neurodegenerative diseases.
Keywords: PDK1 (PDPK1); PDK4; ROCK; neurodegenerative diseases; phosphorylation; sialylation; signaling; α-Secretases.
Copyright © 2024 Bizingre, Bianchi, Baudry, Alleaume-Butaux, Schneider and Pietri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Abd-Elrahman K. S., Albaker A., de Souza J. M., Ribeiro F. M., Schlossmacher M. G., Tiberi M., et al. . (2020). Aβ oligomers induce pathophysiological mGluR5 signaling in Alzheimer’s disease model mice in a sex-selective manner. Sci. Signal. 13:eabd2494. doi: 10.1126/scisignal.abd2494, PMID: - DOI - PubMed
-
- Alleaume-Butaux A., Nicot S., Pietri M., Baudry A., Dakowski C., Tixador P., et al. . (2015). Double-edge sword of sustained ROCK activation in prion diseases through Neuritogenesis defects and prion accumulation. PLoS Pathog. 11:e1005073. doi: 10.1371/journal.ppat.1005073, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

