Acetaminophen for Patent Ductus Arteriosus and Risk of Mortality and Pulmonary Morbidity
- PMID: 39011550
- PMCID: PMC11291959
- DOI: 10.1542/peds.2023-065056
Acetaminophen for Patent Ductus Arteriosus and Risk of Mortality and Pulmonary Morbidity
Abstract
Objective: Emerging data indicate that acetaminophen may adversely affect lung health. We examined whether acetaminophen compared with cyclooxygenase (COX) inhibitor alone for patent ductus arteriosus (PDA) is associated with mortality or respiratory morbidity in extremely preterm infants.
Methods: This is a retrospective cohort study using data from the National Institute of Child Health and Human Development Neonatal Research Network. Infants were born at 22 to 28 weeks' gestation or weighing 401 to 1000 g between 2016 and 2020 and received acetaminophen, ibuprofen, and/or indomethacin for PDA closure. The primary outcome was death or grade 2 to 3 bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age. Secondary outcomes included predischarge mortality and respiratory morbidities. Risk ratios were adjusted for baseline and early postnatal factors. Additional exploratory analyses were adjusted for later postnatal covariates.
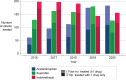
Results: Of 1921 infants, 627 (32.6%) received acetaminophen and 1294 (67.3%) received COX inhibitor only. Multidrug therapy (42.9% vs 4.7%) and surgical or catheter PDA closure (26.5% vs 19.9%) were more common among acetaminophen-exposed infants. Death or grade 2 to 3 BPD at 36 weeks' postmenstrual age was similar between infants treated with acetaminophen versus COX inhibitor only (57.1% vs 58.3%; adjusted relative risk [aRR] 0.96, 95% confidence interval [CI] 0.87-1.06). Acetaminophen was associated with increased risk of predischarge mortality (13.3% vs 10.0%) when adjusting for perinatal and early postnatal factors (aRR 1.42, 95% CI 1.02-1.93), but not in exploratory analyses that included later postnatal factors (aRR 1.28, 95% CI 0.91-1.82).
Conclusions: Treatment with acetaminophen versus COX inhibitor alone for PDA was not associated with the composite outcome of death or BPD in extremely preterm infants. Our results support further evaluation of whether acetaminophen for PDA increases mortality.
Copyright © 2024 by the American Academy of Pediatrics.
Conflict of interest statement
Figures

References
-
- Benitz WE, Bhombal S. The use of non-steroidal anti-inflammatory drugs for patent ductus arteriosus closure in preterm infants. Semin Fetal Neonatal Med. 2017;22(5):302–307 - PubMed
-
- Hammerman C, Bin-Nun A, Markovitch E, et al. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics. 2011;128(6):e1618–e1621 - PubMed
-
- Dowd LA, Wheeler BJ, Al-Sallami HS, et al. Paracetamol treatment for patent ductus arteriosus: practice and attitudes in Australia and New Zealand. J Matern Fetal Neonatal Med. 2019;32(18):3039–3044 - PubMed
-
- Iacobelli S, Lorrain S, Gouyon B, et al. Drug exposure for PDA closure in France: a prospective, cohort-based, analysis. Eur J Clin Pharmacol. 2020;76(12):1765–1772 - PubMed
MeSH terms
Substances
Grants and funding
- U10 HD021373/HD/NICHD NIH HHS/United States
- L40 HD099827/HD/NICHD NIH HHS/United States
- UG1 HD087226/HD/NICHD NIH HHS/United States
- UG1 HD068270/HD/NICHD NIH HHS/United States
- R01 HD107700/HD/NICHD NIH HHS/United States
- UG1 HD027856/HD/NICHD NIH HHS/United States
- UG1 HD034216/HD/NICHD NIH HHS/United States
- U10 HD027871/HD/NICHD NIH HHS/United States
- UG1 HD027904/HD/NICHD NIH HHS/United States
- UG1 HD027880/HD/NICHD NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- UG1 HD053109/HD/NICHD NIH HHS/United States
- UG1 HD027851/HD/NICHD NIH HHS/United States
- U10 HD036790/HD/NICHD NIH HHS/United States
- UG1 HD068263/HD/NICHD NIH HHS/United States
- K23 HL136843/HL/NHLBI NIH HHS/United States
- U10 HD053119/HD/NICHD NIH HHS/United States
- U01 HD036790/HD/NICHD NIH HHS/United States
- F32 HD098782/HD/NICHD NIH HHS/United States
- UG1 HD068244/HD/NICHD NIH HHS/United States
- UG1 HD027853/HD/NICHD NIH HHS/United States
- UG1 HD112093/HD/NICHD NIH HHS/United States
- UG1 HD087229/HD/NICHD NIH HHS/United States
- UG1 HD040689/HD/NICHD NIH HHS/United States
- UG1 HD053089/HD/NICHD NIH HHS/United States
- R01 HL168066/HL/NHLBI NIH HHS/United States
- UG1 HD068284/HD/NICHD NIH HHS/United States
- UG1 HD021385/HD/NICHD NIH HHS/United States
- UG1 HD040492/HD/NICHD NIH HHS/United States
- UG1 HD021364/HD/NICHD NIH HHS/United States
- UG1 HD068278/HD/NICHD NIH HHS/United States
- U24 HD095254/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources

