Transcriptional responses of Neisseria gonorrhoeae to glucose and lactate: implications for resistance to oxidative damage and biofilm formation
- PMID: 39012148
- PMCID: PMC11323468
- DOI: 10.1128/mbio.01761-24
Transcriptional responses of Neisseria gonorrhoeae to glucose and lactate: implications for resistance to oxidative damage and biofilm formation
Abstract
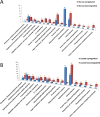
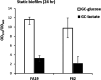
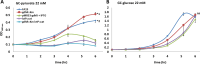
Understanding how bacteria adapt to different environmental conditions is crucial for advancing knowledge regarding pathogenic mechanisms that operate during infection as well as efforts to develop new therapeutic strategies to cure or prevent infections. Here, we investigated the transcriptional response of Neisseria gonorrhoeae, the causative agent of gonorrhea, to L-lactate and glucose, two important carbon sources found in the host environment. Our study revealed extensive transcriptional changes that gonococci make in response to L-lactate, with 37% of the gonococcal transcriptome being regulated, compared to only 9% by glucose. We found that L-lactate induces a transcriptional program that would negatively impact iron transport, potentially limiting the availability of labile iron, which would be important in the face of the multiple hydrogen peroxide attacks encountered by gonococci during its lifecycle. Furthermore, we found that L-lactate-mediated transcriptional response promoted aerobic respiration and dispersal of biofilms, contrasting with an anaerobic condition previously reported to favor biofilm formation. Our findings suggest an intricate interplay between carbon metabolism, iron homeostasis, biofilm formation, and stress response in N. gonorrhoeae, providing insights into its pathogenesis and identifying potential therapeutic targets.IMPORTANCEGonorrhea is a prevalent sexually transmitted infection caused by the human pathogen Neisseria gonorrhoeae, with ca. 82 million cases reported worldwide annually. The rise of antibiotic resistance in N. gonorrhoeae poses a significant public health threat, highlighting the urgent need for alternative treatment strategies. By elucidating how N. gonorrhoeae responds to host-derived carbon sources such as L-lactate and glucose, this study offers insights into the metabolic adaptations crucial for bacterial survival and virulence during infection. Understanding these adaptations provides a foundation for developing novel therapeutic approaches targeting bacterial metabolism, iron homeostasis, and virulence gene expression. Moreover, the findings reported herein regarding biofilm formation and L-lactate transport and metabolism contribute to our understanding of N. gonorrhoeae pathogenesis, offering potential avenues for preventing and treating gonorrhea infections.
Keywords: H2O2 resistance; Neisseria gonorrhoeae; iron transport; lactate utilization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Transcriptome Analysis of Neisseria gonorrhoeae during Natural Infection Reveals Differential Expression of Antibiotic Resistance Determinants between Men and Women.mSphere. 2018 Jun 27;3(3):e00312-18. doi: 10.1128/mSphereDirect.00312-18. Print 2018 Aug 29. mSphere. 2018. PMID: 29950382 Free PMC article.
-
The ironclad truth: how in vivo transcriptomics and in vitro mechanistic studies shape our understanding of Neisseria gonorrhoeae gene regulation during mucosal infection.Pathog Dis. 2017 Jul 31;75(5):ftx057. doi: 10.1093/femspd/ftx057. Pathog Dis. 2017. PMID: 28520925 Free PMC article. Review.
-
Control of gdhR Expression in Neisseria gonorrhoeae via Autoregulation and a Master Repressor (MtrR) of a Drug Efflux Pump Operon.mBio. 2017 Apr 11;8(2):e00449-17. doi: 10.1128/mBio.00449-17. mBio. 2017. PMID: 28400529 Free PMC article.
-
The Gonococcal Transcriptome during Infection of the Lower Genital Tract in Women.PLoS One. 2015 Aug 5;10(8):e0133982. doi: 10.1371/journal.pone.0133982. eCollection 2015. PLoS One. 2015. PMID: 26244506 Free PMC article.
-
Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future.Ann N Y Acad Sci. 2011 Aug;1230:E19-28. doi: 10.1111/j.1749-6632.2011.06215.x. Ann N Y Acad Sci. 2011. PMID: 22239555 Free PMC article. Review.
Cited by
-
Coordinated Transcriptional Increases in Cell Wall Synthesis Genes in Neisseria gonorrhoeae Lacking the Lytic Transglycosylase, ltgA.Curr Microbiol. 2025 May 6;82(6):278. doi: 10.1007/s00284-025-04261-7. Curr Microbiol. 2025. PMID: 40327097 Free PMC article.
References
-
- Bachmann L. 2022. CDC’s 2022 STI surveillance report underscores that STIs must be a public health priority, U.S.D.o.H.a.H. services. In Sexually transmitted infections surveillance. Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
