Monitoring mouse papillomavirus-associated cancer development using longitudinal Pap smear screening
- PMID: 39012151
- PMCID: PMC11323795
- DOI: 10.1128/mbio.01420-24
Monitoring mouse papillomavirus-associated cancer development using longitudinal Pap smear screening
Abstract
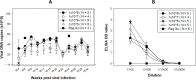
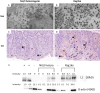
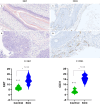
A substantial percentage of the population remains at risk for cervical cancer due to pre-existing human papillomavirus (HPV) infections, despite prophylactic vaccines. Early diagnosis and treatment are crucial for better disease outcomes. The development of new treatments heavily relies on suitable preclinical model systems. Recently, we established a mouse papillomavirus (MmuPV1) model that is relevant to HPV genital pathogenesis. In the current study, we validated the use of Papanicolaou (Pap) smears, a valuable early diagnostic tool for detecting HPV cervical cancer, to monitor disease progression in the MmuPV1 mouse model. Biweekly cervicovaginal swabs were collected from the MmuPV1-infected mice for viral DNA quantitation and cytology assessment. The Pap smear slides were evaluated for signs of epithelial cell abnormalities using the 2014 Bethesda system criteria. Tissues from the infected mice were harvested at various times post-viral infection for additional histological and virological assays. Over time, increased viral replication was consistent with higher levels of viral DNA, and it coincided with an uptick in epithelial cell abnormalities with higher severity scores noted as early as 10 weeks after viral infection. The cytological results also correlated with the histological evaluation of tissues harvested simultaneously. Both immunocompromised and immunocompetent mice with squamous cell carcinoma (SCC) cytology also developed vaginal SCCs. Notably, samples from the MmuPV1-infected mice exhibited similar cellular abnormalities compared to the corresponding human samples at similar disease stages. Hence, Pap smear screening proves to be an effective tool for the longitudinal monitoring of disease progression in the MmuPV1 mouse model.
Importance: Papanicolaou (Pap) smear has saved millions of women's lives as a valuable early screening tool for detecting human papillomavirus (HPV) cervical precancers and cancer. However, more than 200,000 women in the United States alone remain at risk for cervical cancer due to pre-existing HPV infection-induced precancers, as there are currently no effective treatments for HPV-associated precancers and cancers other than invasive procedures including a loop electrosurgical excision procedure (LEEP) to remove abnormal tissues. In the current study, we validated the use of Pap smears to monitor disease progression in our recently established mouse papillomavirus model. To the best of our knowledge, this is the first study that provides compelling evidence of applying Pap smears from cervicovaginal swabs to monitor disease progression in mice. This HPV-relevant cytology assay will enable us to develop and test novel antiviral and anti-tumor therapies using this model to eliminate HPV-associated diseases and cancers.
Keywords: 2014 Bethesda system; HSIL; LSIL; Pap smear; RNAscope; cytology; immunohistochemistry (IHC); in situ hybridization; longitudinal; lower genital infection; mouse model; qPCR; squamous cell carcinoma; the mouse papillomavirus (MmuPV1); transmission electron microscope (TEM); viral copy number.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
- R21 CA274265/CA/NCI NIH HHS/United States
- R21 DE028650/DE/NIDCR NIH HHS/United States
- R21AI121822/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 CA266050/CA/NCI NIH HHS/United States
- 52250/322744/Canadian Government | Canadian Institutes of Health Research (CIHR)
- the Jake Gittlen Memorial Golf Tournament
- R01CA266050/HHS | NIH | National Cancer Institute (NCI)
- the pathology department research initiative fund
- 1R21DE028650/HHS | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD)
- R21 AI121822/AI/NIAID NIH HHS/United States
- R21 CA271069/CA/NCI NIH HHS/United States
- MRT-CDAA-159236/Canadian Government | Canadian Institutes of Health Research (CIHR)
- MRT-168046/Canadian Government | Canadian Institutes of Health Research (CIHR)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
