Multidimensional Analysis of the Adult Human Heart in Health and Disease Using Hierarchical Phase-Contrast Tomography
- PMID: 39012246
- PMCID: PMC11303834
- DOI: 10.1148/radiol.232731
Multidimensional Analysis of the Adult Human Heart in Health and Disease Using Hierarchical Phase-Contrast Tomography
Abstract
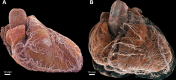
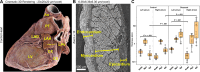
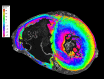
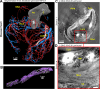
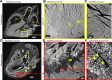
Background Current clinical imaging modalities such as CT and MRI provide resolution adequate to diagnose cardiovascular diseases but cannot depict detailed structural features in the heart across length scales. Hierarchical phase-contrast tomography (HiP-CT) uses fourth-generation synchrotron sources with improved x-ray brilliance and high energies to provide micron-resolution imaging of intact adult organs with unprecedented detail. Purpose To evaluate the capability of HiP-CT to depict the macro- to microanatomy of structurally normal and abnormal adult human hearts ex vivo. Materials and Methods Between February 2021 and September 2023, two adult human donor hearts were obtained, fixed in formalin, and prepared using a mixture of crushed agar in a 70% ethanol solution. One heart was from a 63-year-old White male without known cardiac disease, and the other was from an 87-year-old White female with a history of multiple known cardiovascular pathologies including ischemic heart disease, hypertension, and atrial fibrillation. Nondestructive ex vivo imaging of these hearts without exogenous contrast agent was performed using HiP-CT at the European Synchrotron Radiation Facility. Results HiP-CT demonstrated the capacity for high-spatial-resolution, multiscale cardiac imaging ex vivo, revealing histologic-level detail of the myocardium, valves, coronary arteries, and cardiac conduction system across length scales. Virtual sectioning of the cardiac conduction system provided information on fatty infiltration, vascular supply, and pathways between the cardiac nodes and adjacent structures. HiP-CT achieved resolutions ranging from gross (isotropic voxels of approximately 20 µm) to microscopic (approximately 6.4-µm voxel size) to cellular (approximately 2.3-µm voxel size) in scale. The potential for quantitative assessment of features in health and disease was demonstrated. Conclusion HiP-CT provided high-spatial-resolution, three-dimensional images of structurally normal and diseased ex vivo adult human hearts. Whole-heart image volumes were obtained with isotropic voxels of approximately 20 µm, and local regions of interest were obtained with resolution down to 2.3-6.4 µm without the need for sectioning, destructive techniques, or exogenous contrast agents. Published under a CC BY 4.0 license Supplemental material is available for this article. See also the editorial by Bluemke and Pourmorteza in this issue.
Conflict of interest statement
Figures

Update of
-
Multidimensional Analysis of the Adult Human Heart in Health and Disease using Hierarchical Phase-Contrast Tomography (HiP-CT).bioRxiv [Preprint]. 2023 Oct 10:2023.10.09.561474. doi: 10.1101/2023.10.09.561474. bioRxiv. 2023. Update in: Radiology. 2024 Jul;312(1):e232731. doi: 10.1148/radiol.232731. PMID: 37873359 Free PMC article. Updated. Preprint.
References
-
- Garcia-Canadilla P , Dejea H , Bonnin A , et al . Complex congenital heart disease associated with disordered myocardial architecture in a midtrimester human fetus . Circ Cardiovasc Imaging 2018. ; 11 ( 10 ): e007753 . - PubMed
-
- Lee L , Genge CE , Cua M , et al . Functional assessment of cardiac responses of adult zebrafish (Danio rerio) to acute and chronic temperature change using high-resolution echocardiography . PLoS One 2016. ; 11 ( 1 ): e0145163 . [Published correction appears in PLoS One 2016;11(2):e0149741.] - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

