Riding the storm: managing cytokine-related toxicities in CAR-T cell therapy
- PMID: 39012374
- PMCID: PMC11252192
- DOI: 10.1007/s00281-024-01013-w
Riding the storm: managing cytokine-related toxicities in CAR-T cell therapy
Abstract
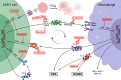
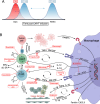
The advent of chimeric antigen receptor T cells (CAR-T) has been a paradigm shift in cancer immunotherapeutics, with remarkable outcomes reported for a growing catalog of malignancies. While CAR-T are highly effective in multiple diseases, salvaging patients who were considered incurable, they have unique toxicities which can be life-threatening. Understanding the biology and risk factors for these toxicities has led to targeted treatment approaches which can mitigate them successfully. The three toxicities of particular interest are cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and immune effector cell-associated hemophagocytic lymphohistiocytosis (HLH)-like syndrome (IEC-HS). Each of these is characterized by cytokine storm and hyperinflammation; however, they differ mechanistically with regard to the cytokines and immune cells that drive the pathophysiology. We summarize the current state of the field of CAR-T-associated toxicities, focusing on underlying biology and how this informs toxicity management and prevention. We also highlight several emerging agents showing promise in preclinical models and the clinic. Many of these established and emerging agents do not appear to impact the anti-tumor function of CAR-T, opening the door to additional and wider CAR-T applications.
Keywords: CAR-T toxicity; Chimeric antigen receptor T cells (CAR-T); cytokine release syndrome (CRS); immune effector cell-assocaited HLH-like syndrome (IEC-HS); immune effector cell-associated neurotoxicity (ICANS).
© 2024. The Author(s).
Conflict of interest statement
DTT serves on advisory boards for Sobi, Jazz, Servier, and BEAM Therapeutics. DTT receives research funding from BEAM Therapeutics and Neoimmune Tech. DTT has patents (US11747346) and patents pending on CAR-T. All other authors have no conflicts of interest to declare.
Figures
References
-
- Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, Olszewska M, Bernal Y, Pegram H, Przybylowski M, Hollyman D, Usachenko Y, Pirraglia D, Hosey J, Santos E et al (2011) Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118(18):4817–4828 10.1182/blood-2011-04-348540 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

