Evaluating the Clinical Impact and Feasibility of Therapeutic Drug Monitoring of Pazopanib in a Real-World Soft-Tissue Sarcoma Cohort
- PMID: 39012619
- PMCID: PMC11271328
- DOI: 10.1007/s40262-024-01399-8
Evaluating the Clinical Impact and Feasibility of Therapeutic Drug Monitoring of Pazopanib in a Real-World Soft-Tissue Sarcoma Cohort
Abstract
Introduction and objective: Pazopanib is registered for metastatic renal cell carcinoma and soft-tissue sarcoma (STS). Its variable pharmacokinetic (PK) characteristics and narrow therapeutic range provide a strong rationale for therapeutic drug monitoring (TDM). Prior studies have defined target levels of drug exposure (≥ 20.5 mg/L) linked to prolonged progression-free survival (PFS), but the added value of using TDM remains unclear. This study investigates the effect of TDM of pazopanib in patients with STS on survival outcomes and dose-limiting toxicities (DLTs) and evaluates the feasibility of TDM-guided dosing.
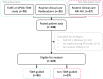
Methods: A TDM-guided cohort was compared to a non-TDM-guided cohort for PFS, overall survival (OS) and DLTs. PK samples were available from all patients, though not acted upon in the non-TDM-guided cohort. We evaluated the feasibility of TDM by comparing the proportion of underdosed patients in our TDM cohort with data from previous publications.
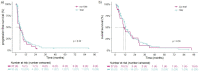
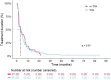
Results: A total of 122 STS patients were included in the TDM-guided cohort (n = 95) and non-TDM-guided cohort (n = 27). The average exposure in the overall population was 30.5 mg/L and was similar in both groups. Median PFS and OS did not differ between the TDM-guided cohort and non-TDM-guided cohort (respectively 5.5 vs 4.4 months, p = 0.3, and 12.6 vs 10.1 months, p = 0.8). Slightly more patients in the non-TDM-guided cohort experienced DLTs (54%) compared to the TDM-guided cohort (44%). The proportion of underdosed patients (13.3%) was halved compared to historical data (26.7%).
Conclusion: TDM reduced the proportion of patients with subtherapeutic exposure levels by ~ 50%. Nonetheless, the added value of TDM for achieving target trough levels of ≥ 20.5 mg/L for pazopanib on survival outcomes could not be confirmed in STS patients.
© 2024. The Author(s).
Conflict of interest statement
MM, ELG, MBAvdK, KW, NADG, RFB, AR, ALTI, H-MO, AV, ML, PH, SLWK, DJARM, KEB, DJT, ADRH, and IMED declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. WTAvdG reports institutional research fees paid to the institute from Lilly and advisory compensation from Springworks, PTC Therapeutics, and Agenus, all outside the submitted work. RHJM reports research funding paid to the institute from Astellas, Bayer, Boehringer-Ingelheim, Cristal Therapeutics, Deuter Oncology, Nordic Pharma, Novartis, Pamgene, Pfizer, Roche, Sanofi, and Servier, all outside the submitted work. HG reports institutional research funding from Daiichi Sankyo, Deciphera Pharmaceuticals, Ipsen, and Novartis. ADRH is an Editorial Board member of
Figures

References
-
- European Medicines Agency. Summary of product characteristics Vortrient 200 and 400 mg film-coated tablets. 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/votrient. Cited 15 Aug 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

