Dactylfungins and Tetralones: Bioactive Metabolites from a Nematode-Associated Laburnicola nematophila
- PMID: 39012621
- PMCID: PMC11287750
- DOI: 10.1021/acs.jnatprod.4c00623
Dactylfungins and Tetralones: Bioactive Metabolites from a Nematode-Associated Laburnicola nematophila
Abstract
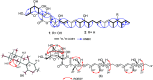
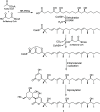
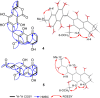
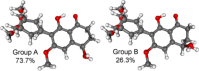
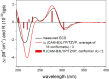
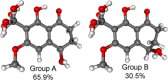
A chemical investigation of Laburnicola nematophila, isolated from cysts of the plant parasitic nematode Heterodera filipjevi, affored three dactylfungin derivatives (1-3) and three tetralone congeners (4-6). Dactylfungin C (1), laburnicolin (4), and laburnicolenone (5) are previously undescribed natural products. Chemical structures of the isolated compounds were determined based on 1D and 2D NMR spectroscopic analyses together with HR-ESI-MS spectrometry and comparison with data reported in the literature. The relative configurations of compounds 1, 2, and 4-6 were determined based on their ROESY data and analysis of their coupling constants (J values). The absolute configurations of 4-6 were determined through the comparison of their measured and calculated TDDFT-ECD spectra. Compounds 1-3 were active against azole-resistant Aspergillus fumigatus.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Hyde K. D.; Al-Hatmi A. M. S.; Andersen B.; Boekhout T.; Buzina W.; Dawson T. L.; Eastwood D. C.; Jones E. B. G.; de Hoog S.; Kang Y.; et al. Fungal Divers. 2018, 93, 161–194. 10.1007/s13225-018-0413-9. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

